Back
BackProblem 1
Step 2 of the iodination of benzene shows water acting as a base and removing a proton from the sigma complex. We did not consider the possibility of water acting as a nucleophile and attacking the carbocation, as in an electrophilic addition to an alkene. Draw the reaction that would occur if water reacted as a nucleophile and added to the carbocation. Explain why this type of addition is rarely observed.
Problem 2
Propose a mechanism for the aluminum chloride–catalyzed reaction of benzene with chlorine.
Problem 3
p-Xylene undergoes nitration much faster than benzene. Use resonance forms of the sigma complex to explain this accelerated rate
Problem 5b
(b) Explain why m-xylene undergoes nitration 100 times faster than p-xylene.
Problem 6
Styrene (vinylbenzene) undergoes electrophilic aromatic substitution much faster than benzene, and the products are found to be primarily ortho- and para-substituted styrenes. Use resonance forms of the intermediates to explain these results.
Problem 7
Propose a mechanism for the bromination of ethoxybenzene to give o- and p-bromoethoxybenzene.
Problem 9
When bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?
Problem 10c
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2,4,6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the yields (mostly m-nitroaniline) are poor.
c. Although nitration of aniline is slow and gives mostly meta substitution, nitration of acetanilide (PhNHCOCH3) goes quickly and gives mostly para substitution. Use resonance forms to explain this difference in reactivity.
Problem 12a,b,c
Predict the mononitration products of the following compounds.
a. o-nitrotoluene
b. m-chlorotoluene
c. o-bromobenzoic acid
Problem 12d
Predict the mononitration products of the following compounds.
d. p-methoxybenzoic acid
Problem 12f
Predict the mononitration products of the following compounds.
f. o-hydroxyacetophenone
Problem 14a(i)
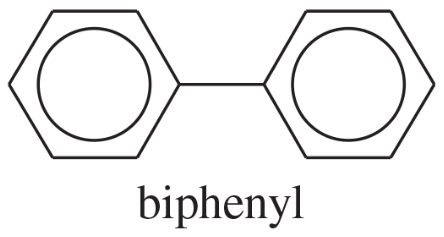
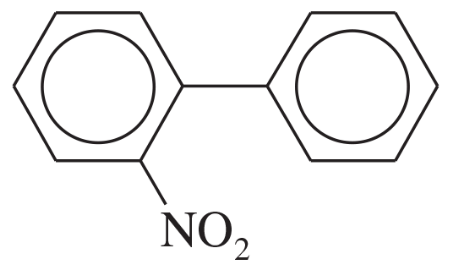
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
a. Use resonance forms of a sigma complex to show why a phenyl substituent should be ortho, para-directing.
(i)
Problem 14a(vi)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
a. Use resonance forms of a sigma complex to show why a phenyl substituent should be ortho, para-directing.
(vi)
Problem 14b(vi)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(vi)
Problem 14b(i)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(i)
Problem 14b(iv)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(iv)
Problem 14b(v)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(v)
Problem 14b(ii)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(ii)
Problem 14b(iii)
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
b. Predict the mononitration products of the following compounds
(iii)
Problem 15a
Propose products (if any) and mechanisms for the following AlCl3-catalyzed reactions:
a. chlorocyclohexane with benzene
Problem 15b
Propose products (if any) and mechanisms for the following AlCl3-catalyzed reactions:
b. methyl chloride with anisole
Problem 15c
Propose products (if any) and mechanisms for the following AlCl3-catalyzed reactions:
c. 3-chloro-2,2-dimethylbutane with isopropylbenzene
Problem 16c
For each reaction, show the generation of the electrophile and predict the products.
c. tert-butylbenzene + 2-methylpropene + HF
Problem 16d
For each reaction, show the generation of the electrophile and predict the products.
d. propan-2-ol + toluene + BF3
Problem 17b
Predict the products (if any) of the following reactions.
b. (excess) toluene + butan-1-ol + BF3
Problem 17c
Predict the products (if any) of the following reactions.
a. (excess) benzene + isobutyl chloride + AlCl3
Problem 17-20e
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
e. 3-methyl-1-phenylbutane
Problem 17-12e
Predict the mononitration products of the following compounds.
e. m-cresol (m-methylphenol)
Problem 18d
Which reactions will produce the desired product in good yield? You may assume that aluminum chloride is added as a catalyst in each case. For the reactions that will not give a good yield of the desired product, predict the major products.
(d) Reagents: benzamide (PhCONH2) + CH3CH2Cl
Desired Product: p-ethylbenzamide
Problem 18e
Which reactions will produce the desired product in good yield? You may assume that aluminum chloride is added as a catalyst in each case. For the reactions that will not give a good yield of the desired product, predict the major products.
(e) Reagents: toluene + HNO3, H2SO4, heat
Desired Product: 2,4,6-trinitrotoluene (TNT)