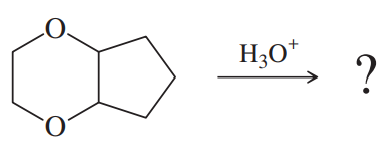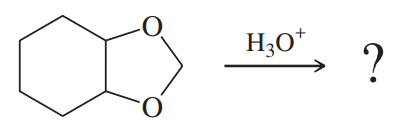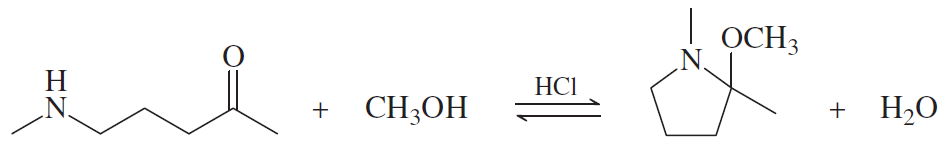Back
BackProblem 64e
Hydration of alkynes (via oxymercuration) gives good yields of single compounds only with symmetrical or terminal alkynes. Show what the products would be from hydration of each compound.
e. 3-methylcyclodecyne
Problem 65a,b,c
Which of the following compounds would give a positive Tollens test? (Remember that the Tollens test involves mild basic aqueous conditions.)
(a) CH3CH2CH2COCH3
(b) CH3CH2CH2CH2CHO
(c) CH3CH=CHCH=CHOH
Problem 65d,e,f
Which of the following compounds would give a positive Tollens test? (Remember that the Tollens test involves mild basic aqueous conditions.)
(d) CH3CH2CH2CH2CH(OH)OCH3
(e) CH3CH2CH2CH2CH(OCH3)2
(f)
Problem 66
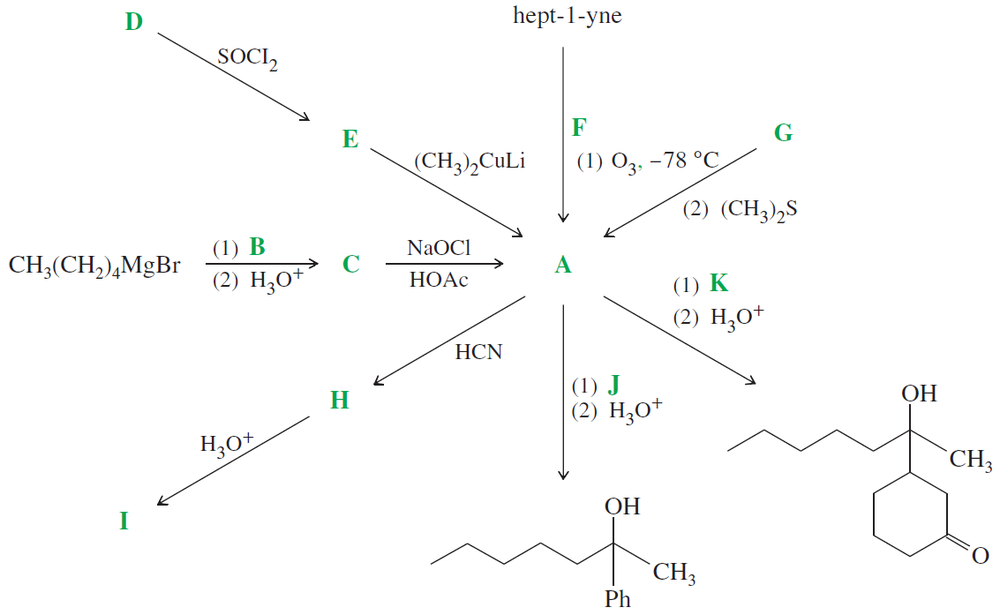
Solving the following road-map problem depends on determining the structure of A, the key intermediate. Give structures for compounds A through K.
Problem 67a
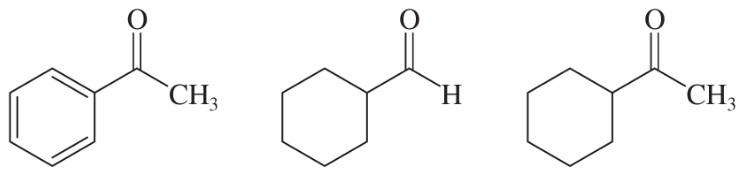
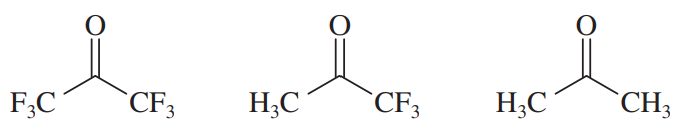
Within each set of structures, indicate which will react fastest, and which slowest, toward nucleophilic addition in basic conditions.
(a)
Problem 67b
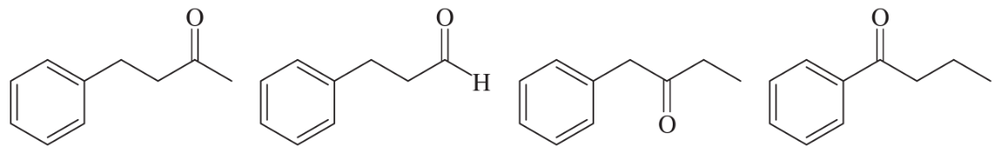
Within each set of structures, indicate which will react fastest, and which slowest, toward nucleophilic addition in basic conditions.
(b)
Problem 67c
Within each set of structures, indicate which will react fastest, and which slowest, toward nucleophilic addition in basic conditions.
(c)
Problem 68a
One of these reacts with dilute aqueous acid and the other does not. Give a mechanism for the one that reacts, and show why this mechanism does not work for the other one.
(a)
Problem 68b
One of these reacts with dilute aqueous acid and the other does not. Give a mechanism for the one that reacts, and show why this mechanism does not work for the other one.
(b)
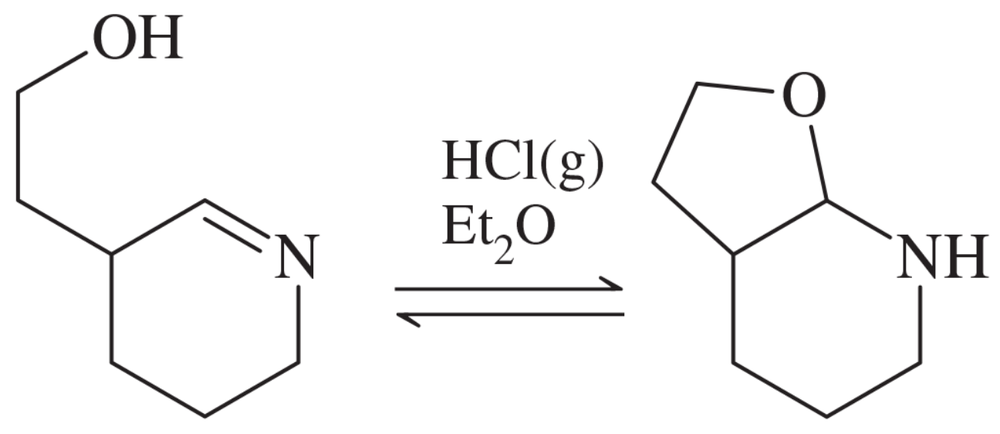
Problem 69
Show a complete mechanism for this equilibrium established in diethyl ether with HCl gas as catalyst.
Problem 70
Show a complete mechanism for this reaction.
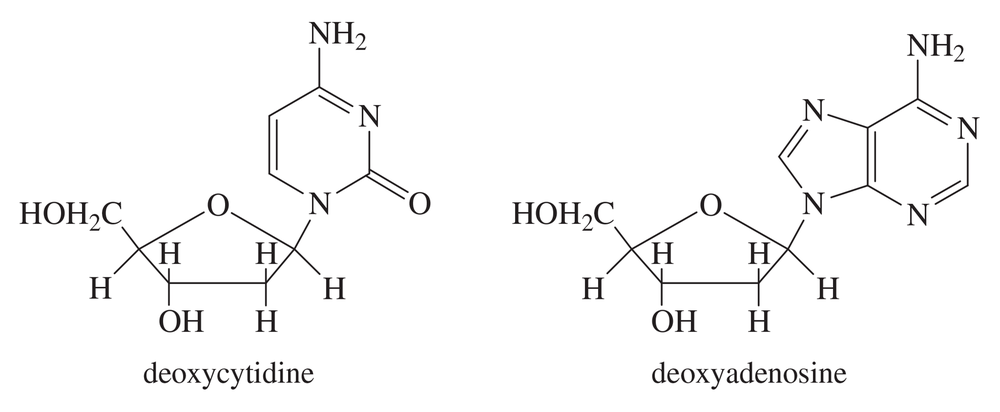
Problem 72
The nucleosides that make up DNA have heterocyclic rings linked to deoxyribose by an aminoacetal functional group. Point out the aminoacetal linkages in deoxycytidine and deoxyadenosine.
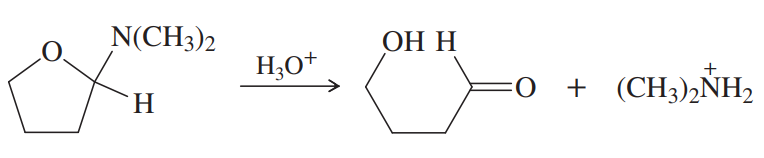
Problem 72a
Simple aminoacetals hydrolyze quickly and easily in dilute acid. Propose a mechanism for hydrolysis of the following aminoacetal: