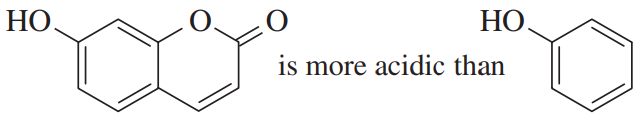Back
BackProblem 12a,b,c
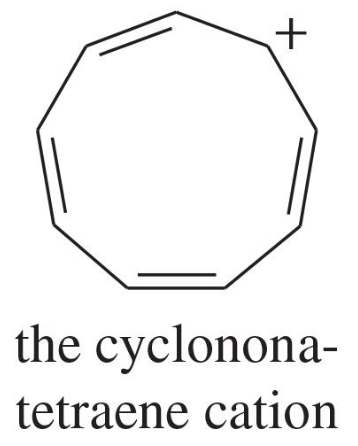
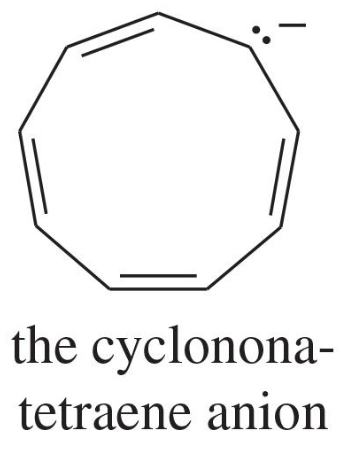
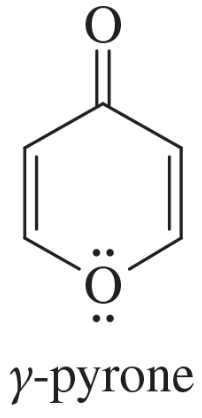
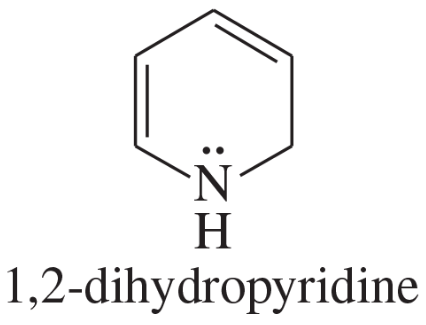
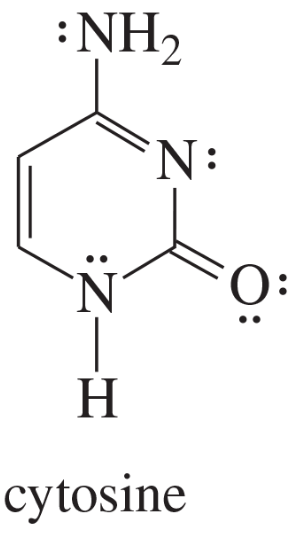
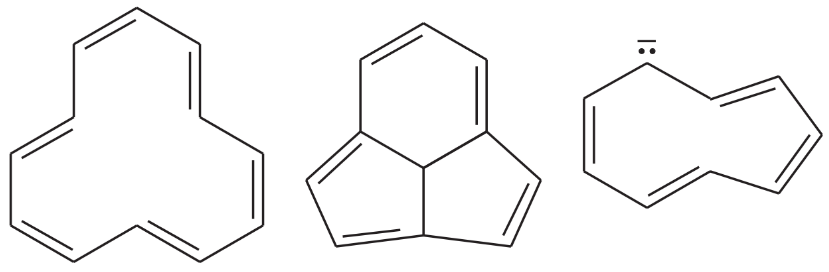
Explain why each compound or ion should be aromatic, antiaromatic, or nonaromatic.
(a)
(b)
(c)
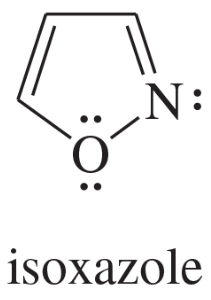
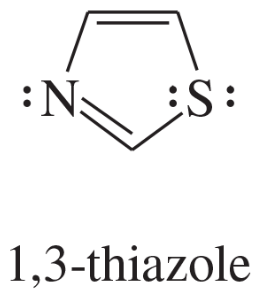
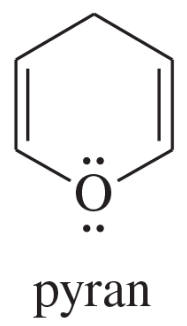
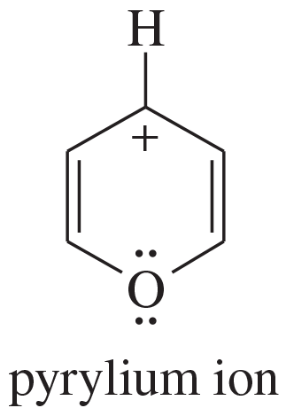
Problem 12d,e,f
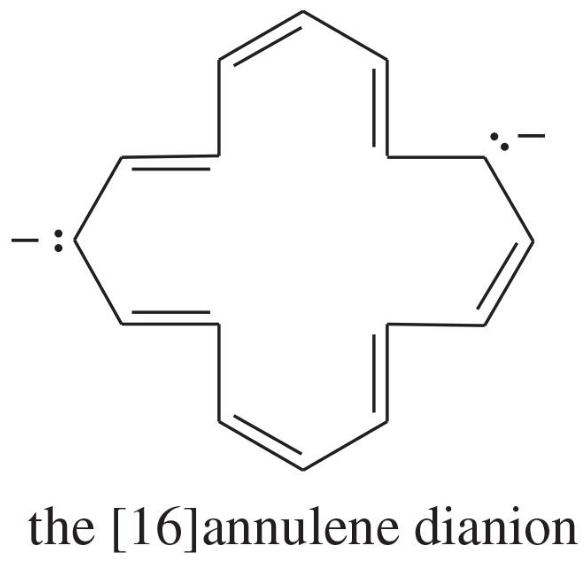
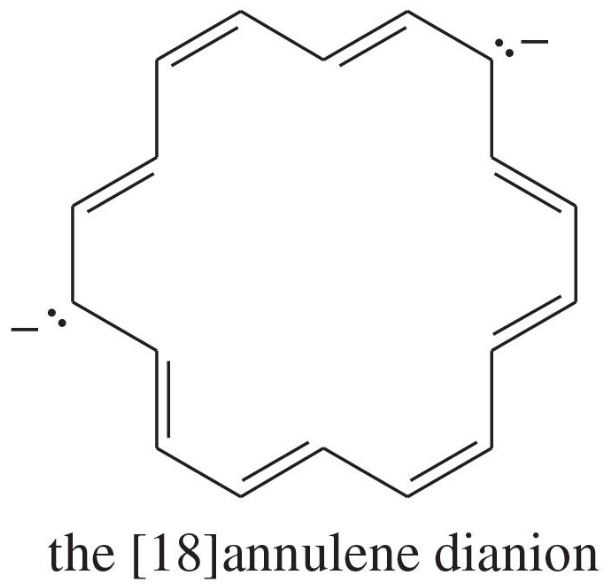
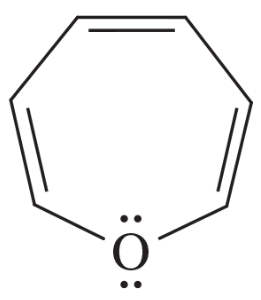
Explain why each compound or ion should be aromatic, antiaromatic, or nonaromatic.
(d)
(e)
(f) the [20]annulene dication
Problem 13
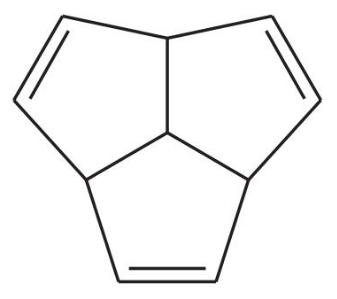
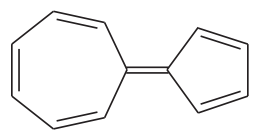
The following hydrocarbon has an unusually large dipole moment. Explain how a large dipole moment might arise.
Problem 14
When 3-chlorocyclopropene is treated with AgBF4, AgCl precipitates. The organic product can be obtained as a crystalline material, soluble in polar solvents such as nitromethane but insoluble in hexane. When the crystalline material is dissolved in nitromethane containing KCl, the original 3-chlorocyclopropene is regenerated. Determine the structure of the crystalline material, and write equations for its formation and its reaction with chloride ion.
Problem 15
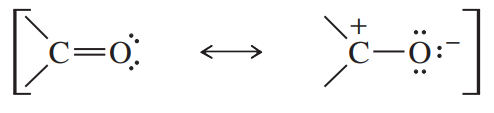
The polarization of a carbonyl group can be represented by a pair of resonance structures:
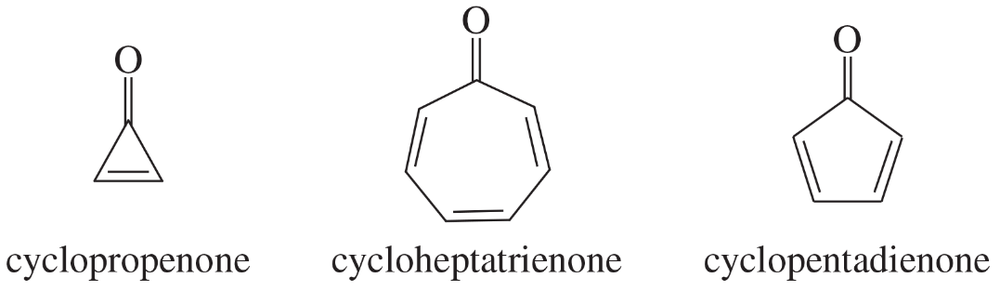
Cyclopropenone and cycloheptatrienone are more stable than anticipated. Cyclopentadienone, however, is relatively unstable and rapidly undergoes a Diels–Alder dimerization. Explain.
Problem 16a,b
a. Explain how pyrrole is isoelectronic with the cyclopentadienyl anion.
b. Specifically, what is the difference between the cyclopentadienyl anion and pyrrole?
Problem 16c
Draw resonance forms to show the charge distribution on the pyrrole structure.
Problem 17
Show which of the nitrogen atoms in purine are basic, and which one is not basic. For the nonbasic nitrogen, explain why its nonbonding electrons are not easily available to become protonated.
Problem 19a,b,c
Explain why each compound is aromatic, antiaromatic, or nonaromatic.
(a)
(b)
(c)
Problem 19d,e,f
Explain why each compound is aromatic, antiaromatic, or nonaromatic.
(d)
(e)
(f)
Problem 19g,h
Explain why each compound is aromatic, antiaromatic, or nonaromatic.
(g)
(h)
Problem 22a
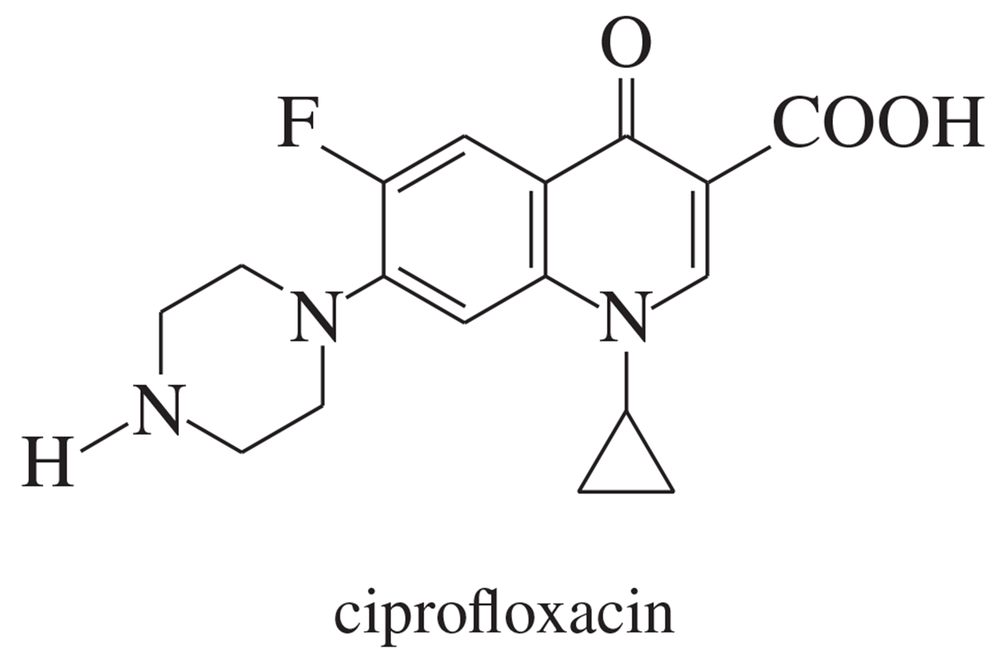
Ciprofloxacin is a member of the fluoroquinolone class of antibiotics.
a. Which of its rings are aromatic?
Problem 22b
Ciprofloxacin is a member of the fluoroquinolone class of antibiotics.
(b) Which nitrogen atoms are basic?
Problem 23
Draw and name all the chlorinated benzenes having from one to six chlorine atoms.
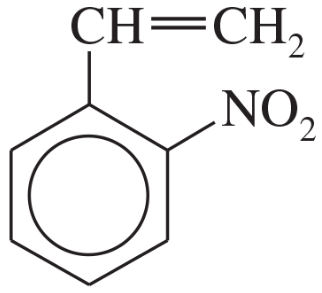
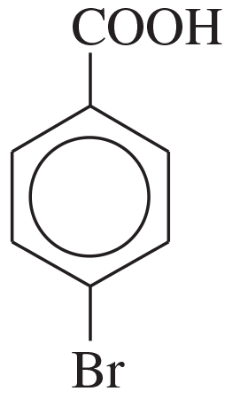
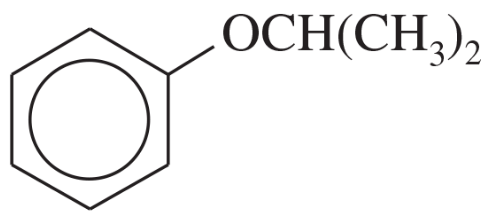
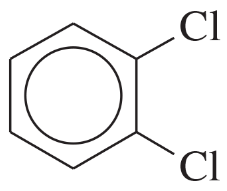
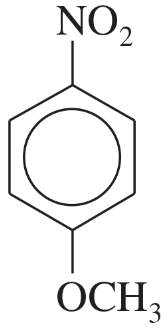
Problem 24a,b,c
Name the following compounds:
(a)
(b)
(c)
Problem 24d,e,f
Name the following compounds:
(d)
(e)
(f)
Problem 25
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer with an intense absorption at 250 nm and a weaker absorption at 290 nm. Suggest a structure for the isomeric product and propose a mechanism for its formation.
Problem 26a,b,c
Draw the structure of each compound.
(a) o-nitroanisole
(b) 2,4-dimethoxyphenol
(c) p-aminobenzoic acid
Problem 26i,j
Draw the structure of each compound.
(i) tropylium chloride
(j) sodium cyclopentadienide
Problem 26m,n
Draw the structure of each compound.
(m) p-toluenesulfonic acid
(n) o-xylene
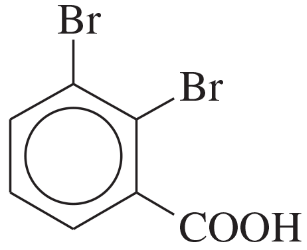
Problem 27a,b,c
Name the following compounds:
(a)
(b)
(c)
Problem 29a,b
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
(a)
(b)
Problem 29c
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
(c)
Problem 29d
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
(d)
Problem 30
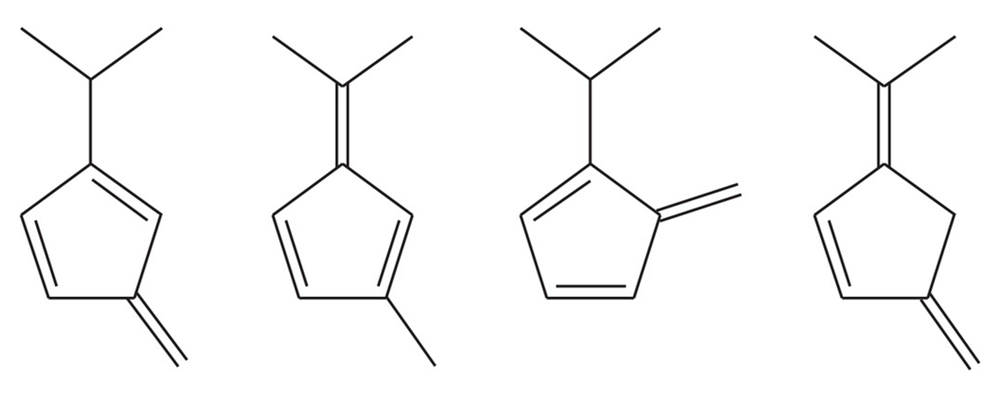
One of the following hydrocarbons is much more acidic than the others. Indicate which one, and explain why it is unusually acidic.
Problem 32a
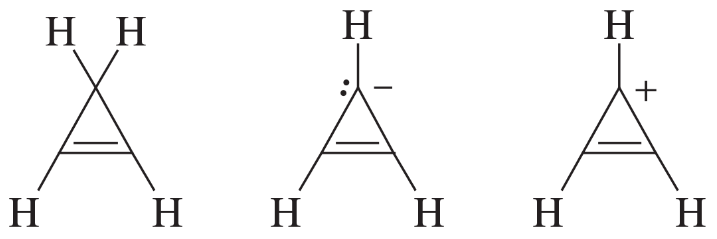
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(a)
Problem 32b
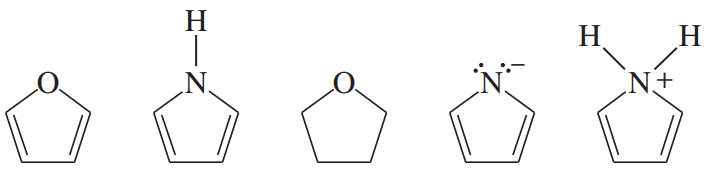
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(b)
Problem 32c
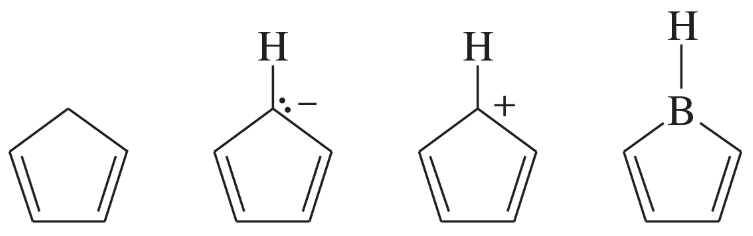
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(c)
Problem 32d
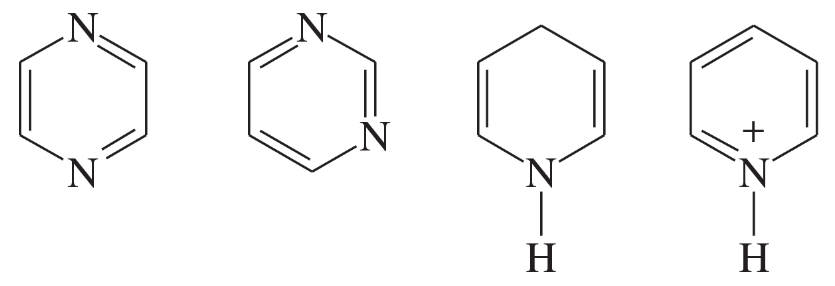
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(d)
Problem 32g
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(g)