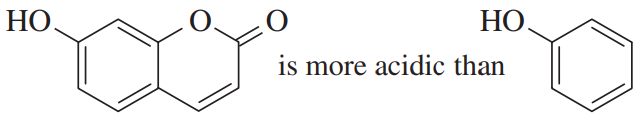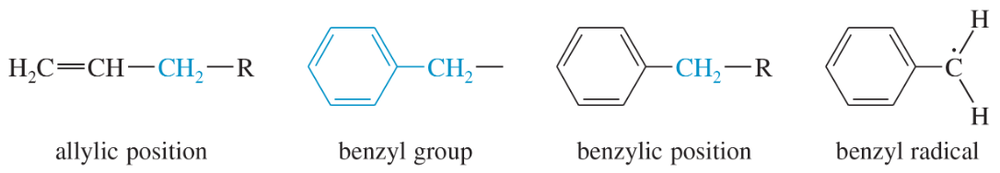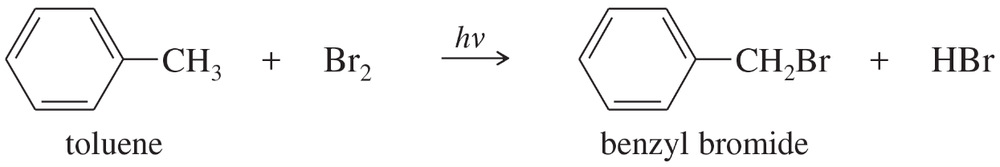Back
BackProblem 25
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer with an intense absorption at 250 nm and a weaker absorption at 290 nm. Suggest a structure for the isomeric product and propose a mechanism for its formation.
Problem 26a,b,c
Draw the structure of each compound.
(a) o-nitroanisole
(b) 2,4-dimethoxyphenol
(c) p-aminobenzoic acid
Problem 26i,j
Draw the structure of each compound.
(i) tropylium chloride
(j) sodium cyclopentadienide
Problem 26m,n
Draw the structure of each compound.
(m) p-toluenesulfonic acid
(n) o-xylene
Problem 27a,b,c
Name the following compounds:
(a)
(b)
(c)
Problem 29a,b
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
(a)
(b)
Problem 29c
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
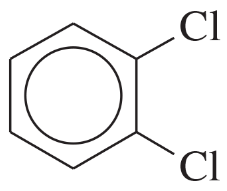
(c)
Problem 29d
Four pairs of compounds are shown. In each pair, one of the compounds reacts more quickly, or with a more favorable equilibrium constant, than the less conjugated system. In each case, explain the enhanced reactivity.
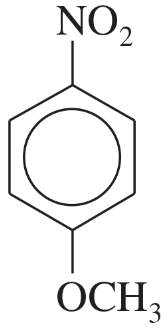
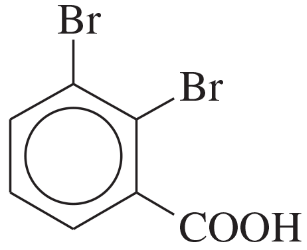
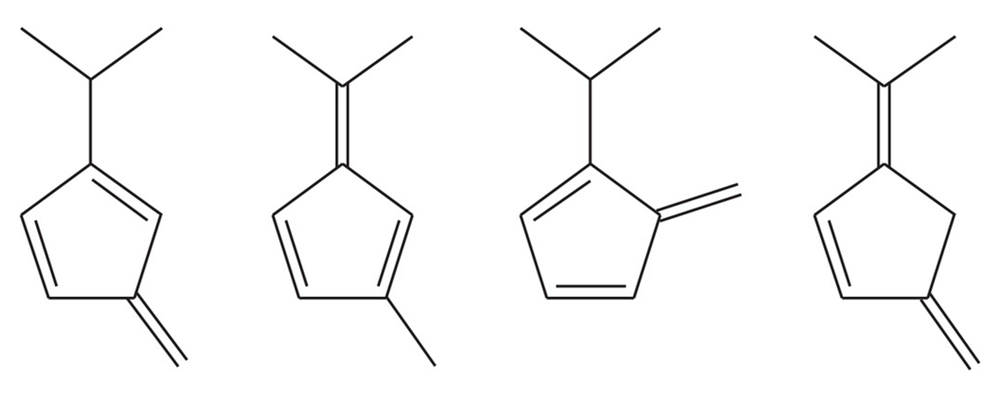
(d)
Problem 30
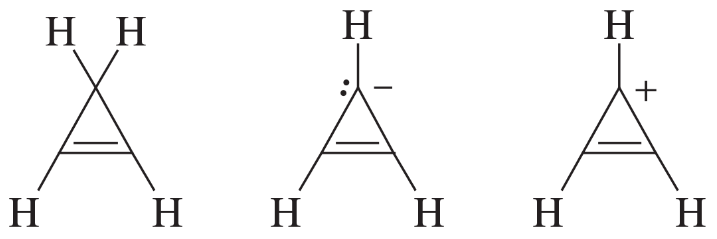
One of the following hydrocarbons is much more acidic than the others. Indicate which one, and explain why it is unusually acidic.
Problem 32a
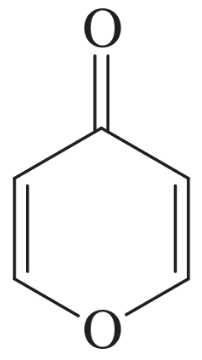
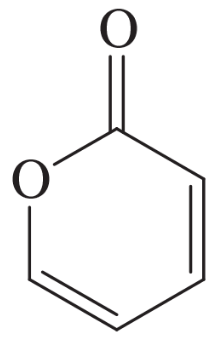
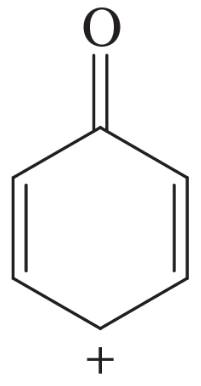
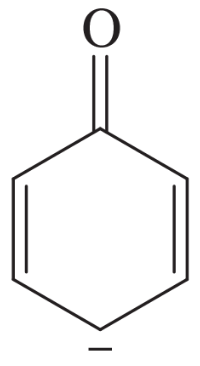
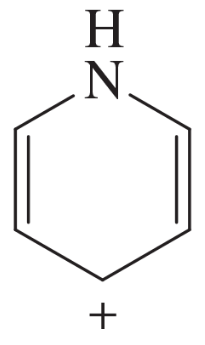
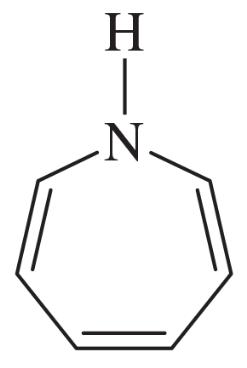
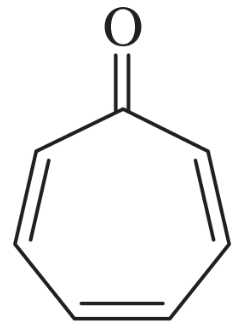
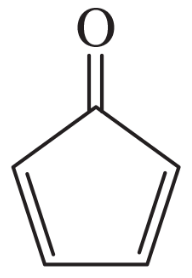
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(a)
Problem 32b
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(b)
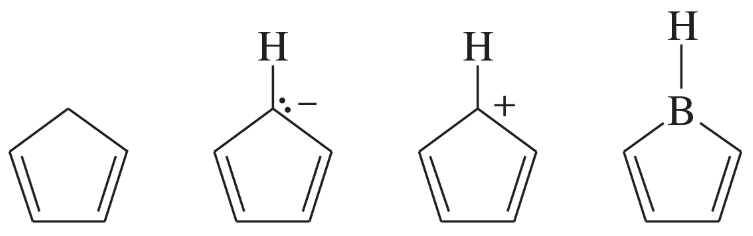
Problem 32c
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(c)
Problem 32d
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(d)
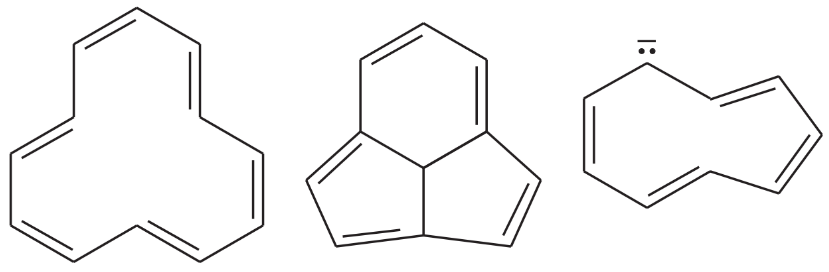
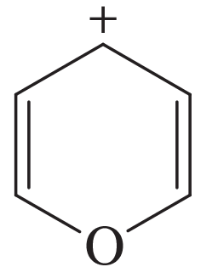
Problem 32g
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons in the ring.
(g)
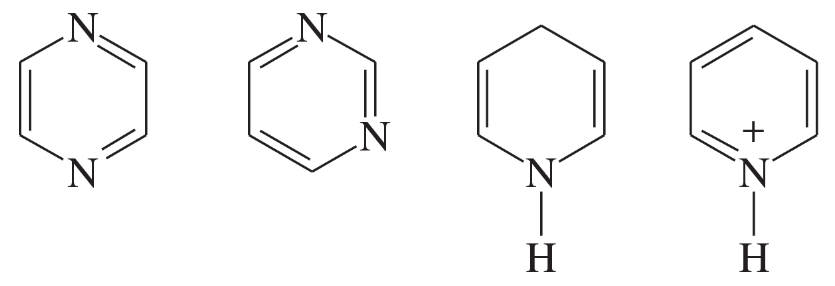
Problem 34a,b,c
Each of the following heterocycles includes one or more nitrogen atoms. Classify each nitrogen atom as strongly basic or weakly basic, according to the availability of its lone pair of electrons.
(a)
(b)
(c)
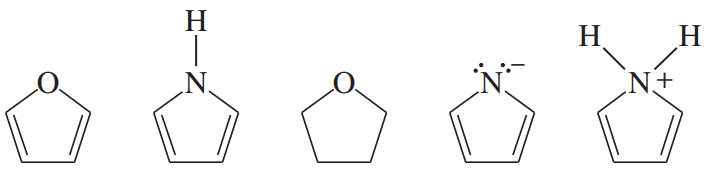
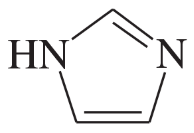
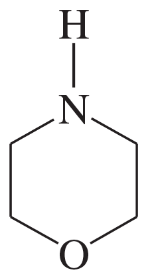
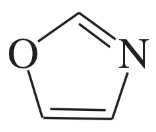
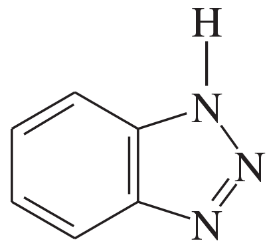
Problem 34d,e
Each of the following heterocycles includes one or more nitrogen atoms. Classify each nitrogen atom as strongly basic or weakly basic, according to the availability of its lone pair of electrons.
(d)
(e)
Problem 35(1)a,b,c
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
(a)
(b)
(c)
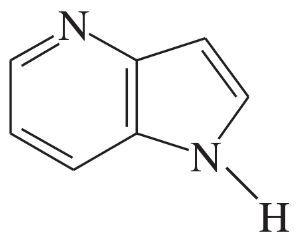
Problem 35d
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
2. Predict which nitrogen atoms are more basic than water and which are less basic.
(d)
Problem 35e
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
2. Predict which nitrogen atoms are more basic than water and which are less basic.
(e)
Problem 35f
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
2. Predict which nitrogen atoms are more basic than water and which are less basic.
(f)
Problem 35m
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
2. Predict which nitrogen atoms are more basic than water and which are less basic.
(m)
Problem 35n,o
Some of the following compounds show aromatic properties, and others do not.
1. Predict which ones are likely to be aromatic, and explain why they are aromatic.
(n)
(o)
Problem 36a(3)
The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
a. Use resonance forms to show the delocalization (over four carbon atoms) of the negative charge of the benzyl anion.
Problem 36a(2)
The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
a. Use resonance forms to show the delocalization (over four carbon atoms) of the unpaired electron of the benzyl radical.
Problem 36a(1)
The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
a. Use resonance forms to show the delocalization (over four carbon atoms) of the positive charge of the benzyl cation.
Problem 36b
The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
b. Toluene reacts with bromine in the presence of light to give benzyl bromide. Propose a mechanism for this reaction.
Problem 36c
The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond alters the reactivity of groups in the allylic position.
Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates.
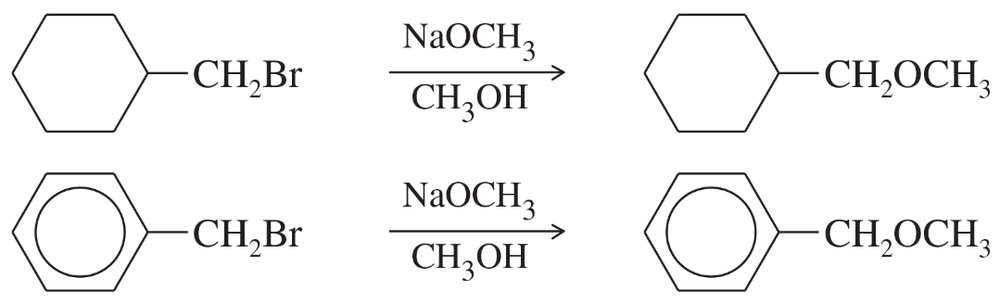
c. Which of the following reactions will have the faster rate and give the better yield? Use a drawing of the transition state to explain your answer.
Problem 37a
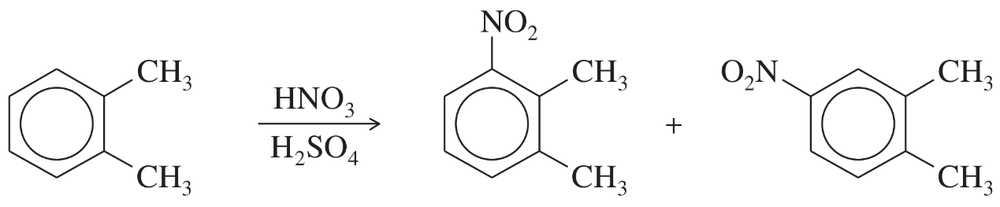
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
a. How many isomers are formed by nitration of m-xylene?
Problem 37b
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
b. How many isomers are formed by nitration of p-xylene?
Problem 37c
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
c. A turn-of-the-century chemist isolated an aromatic compound of molecular formula C6H4Br2. He carefully nitrated this compound and purified three isomers of formula C6H3Br2NO2. Propose structures for the original compound and the three nitrated derivatives