Back
BackProblem 38
For each NMR spectrum, propose a structure consistent with the spectrum and the additional information provided.
<IMAGE>
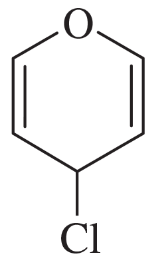
a. Elemental analysis shows the molecular formula to be C8H7OCl. The IR spectrum shows a moderate absorption at 1602 cm–1 and a strong absorption at 1690 cm–1.
b. The mass spectrum shows a double molecular ion of ratio 1:1 at m/z 184 and 186.
<IMAGE>
Problem 39b
Recall (Section 16-10) that two positions of anthracene sometimes react more like polyenes than like aromatic compounds.
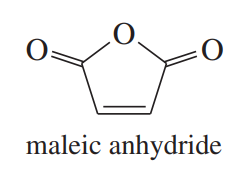
b. The Diels–Alder reaction of anthracene with maleic anhydride is a common organic lab experiment. Predict the product of this Diels–Alder reaction.
Problem 40a,b
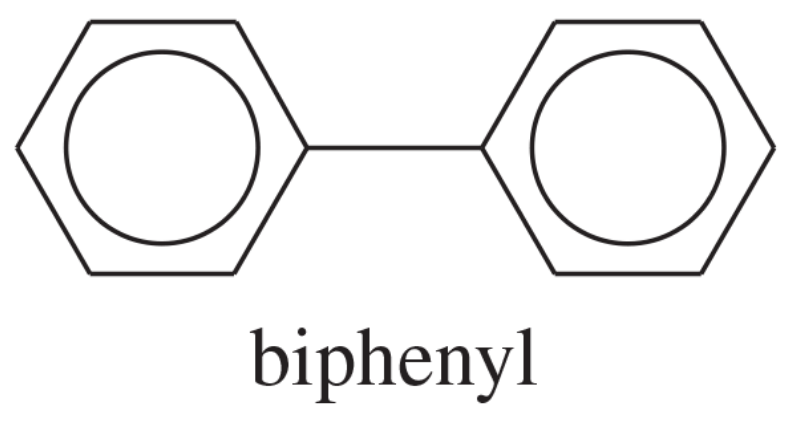
Biphenyl has the following structure.
a. Is biphenyl a (fused) polynuclear aromatic hydrocarbon?
b. How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?
Problem 40c,d
Biphenyl has the following structure.
c. The heat of hydrogenation for biphenyl is about 418 kJ/mol (100 kcal/mol). Calculate the resonance energy of biphenyl.
d. Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain the difference in the resonance energies of naphthalene and biphenyl.
Problem 41
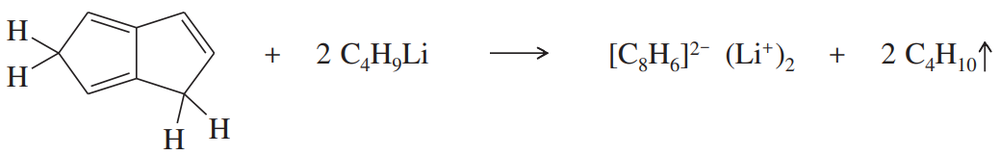
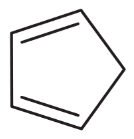
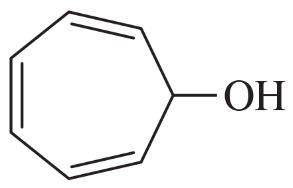
Anions of hydrocarbons are rare, and dianions of hydrocarbons are extremely rare. The following hydrocarbon reacts with two equivalents of butyllithium to form a dianion of formula [C8H6]2–. Propose a structure for this dianion, and suggest why it forms so readily.
Problem 42a,b,c
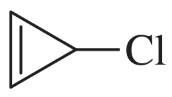
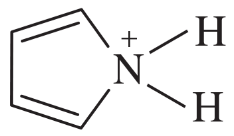
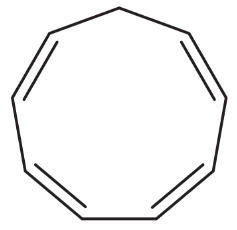
How would you convert the following compounds to aromatic compounds?
(a)
(b)
(c)
Problem 42d,e,f
How would you convert the following compounds to aromatic compounds?
(d)
(e)
(f)
Problem 43a
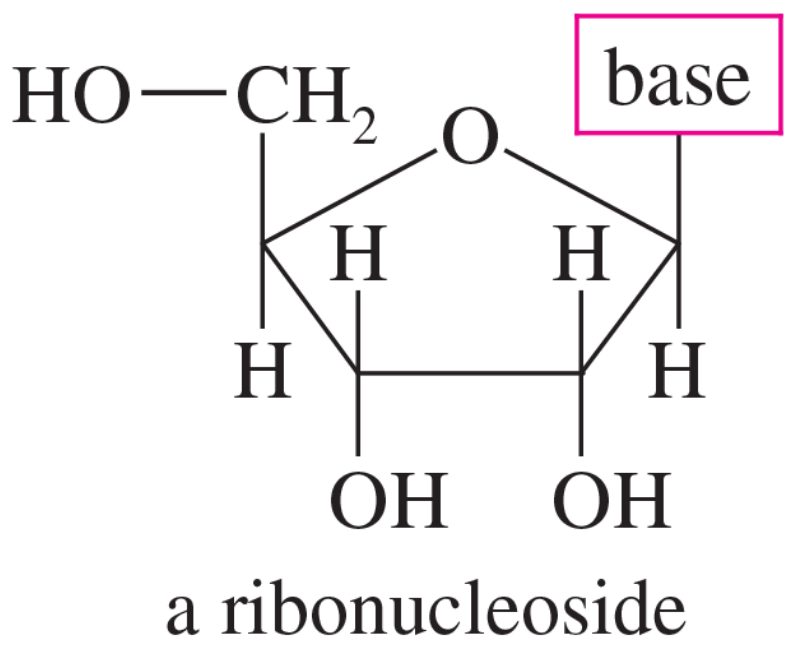
The ribonucleosides that make up ribonucleic acid (RNA) are composed of D-ribose (a sugar) and four heterocyclic “bases.” The general structure of a ribonucleoside is shown here.
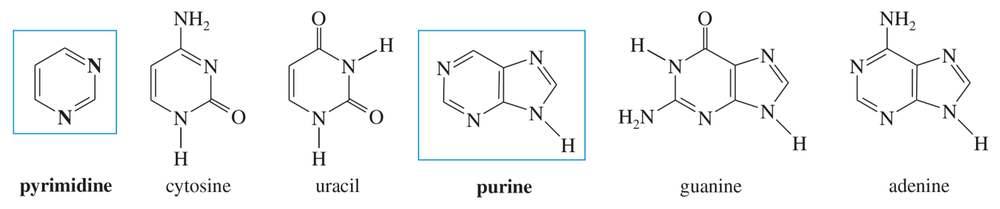
The four heterocyclic bases are cytosine, uracil, guanine, and adenine. Cytosine and uracil are called pyrimidine bases because their structures resemble pyrimidine. Guanine and adenine are called purine bases because their structures resemble purine.
a. Determine which rings of these bases are aromatic.
Problem 43b
The ribonucleosides that make up ribonucleic acid (RNA) are composed of D-ribose (a sugar) and four heterocyclic “bases.” The general structure of a ribonucleoside is shown here.
The four heterocyclic bases are cytosine, uracil, guanine, and adenine. Cytosine and uracil are called pyrimidine bases because their structures resemble pyrimidine. Guanine and adenine are called purine bases because their structures resemble purine.
b. Predict which nitrogen atoms are basic.
Problem 43c
The ribonucleosides that make up ribonucleic acid (RNA) are composed of D-ribose (a sugar) and four heterocyclic “bases.” The general structure of a ribonucleoside is shown here.
The four heterocyclic bases are cytosine, uracil, guanine, and adenine. Cytosine and uracil are called pyrimidine bases because their structures resemble pyrimidine. Guanine and adenine are called purine bases because their structures resemble purine.
c. Do any of these bases have easily formed tautomers that are aromatic? (Consider moving a proton from nitrogen to a carbonyl group to form a phenolic derivative.)
Problem 44a,b
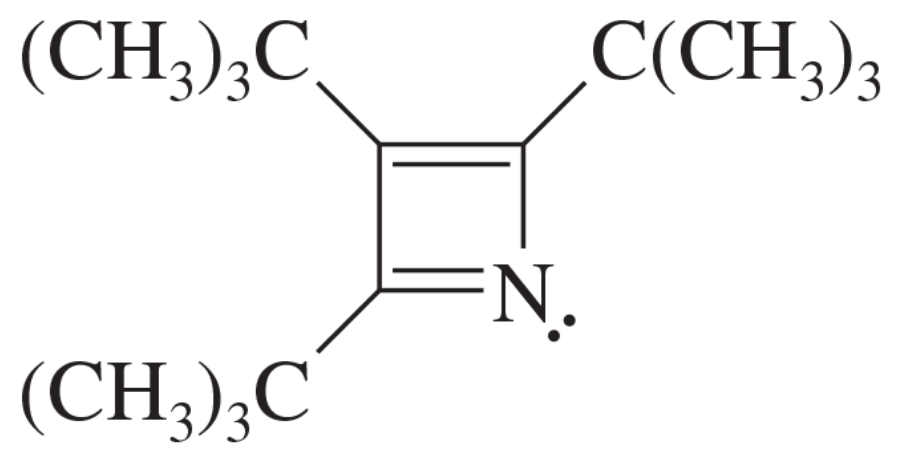
Consider the following compound, which has been synthesized and characterized:
a. Assuming this molecule is entirely conjugated, do you expect it to be aromatic, antiaromatic, or nonaromatic?
b. Why was this molecule synthesized with three tert-butyl substituents? Why not make the unsubstituted compound and study it instead?
Problem 46
An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how it is consistent with the spectra. Show the fragmentations that give the prominent peaks at m/z 127 and 155 in the mass spectrum.
<IMAGES>
Problem 47
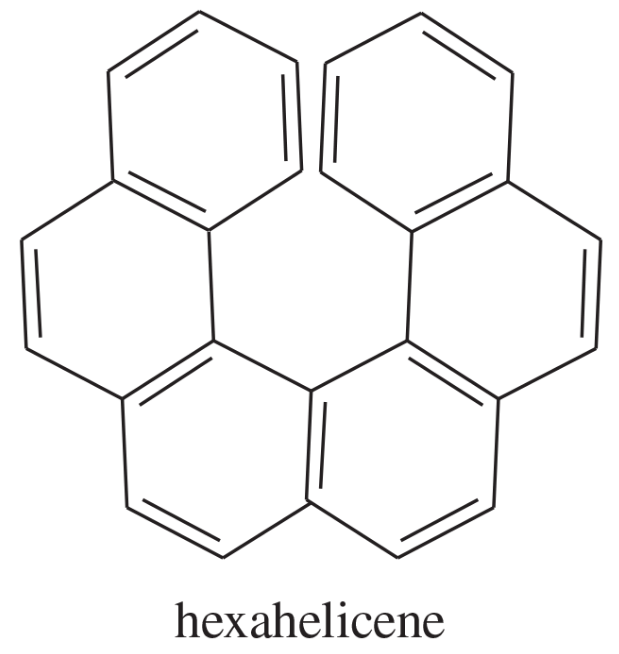
Hexahelicene seems a poor candidate for optical activity because all its carbon atoms are sp2 hybrids and presumably flat. Nevertheless, hexahelicene has been synthesized and separated into enantiomers. Its optical rotation is enormous: [α]D = 3700°. Explain why hexahelicene is optically active, and speculate as to why the rotation is so large.
Problem 50
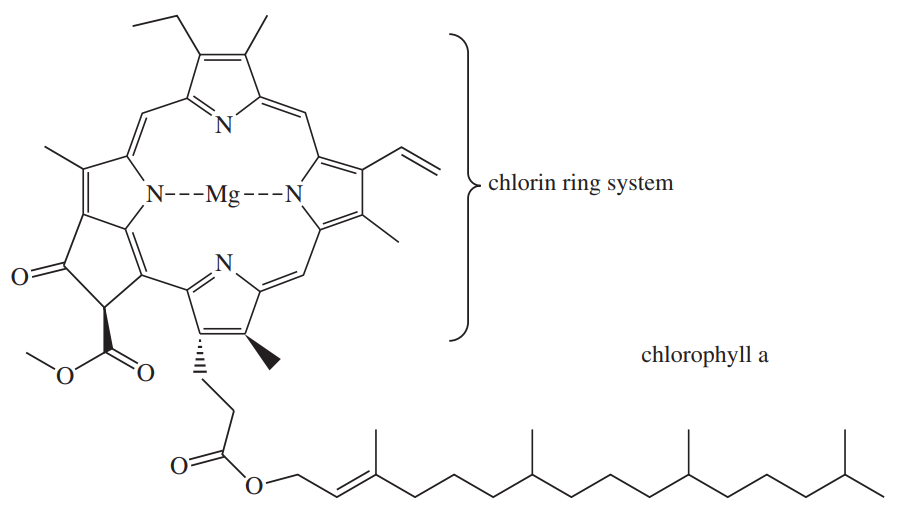
Chlorophyll is the general name for a family of compounds present in algae and green plants. These molecules use the energy in sunlight to convert carbon dioxide and water into carbohydrates and other energy sources. At the heart of chlorophyll (shown below) is a large-ring magnesium complex called a chlorin. Circle each double bond in the large cyclic conjugated pi system that makes it aromatic. How many pi electrons are in this aromatic system?