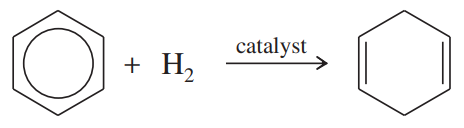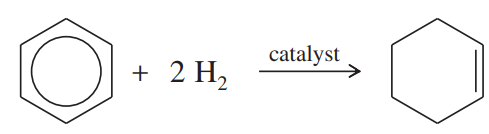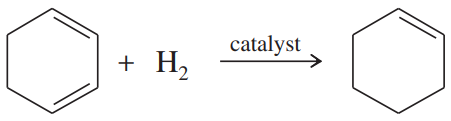Back
BackProblem 2a
Using the information in Figure 16-2, calculate the values of ∆H° for the following reactions:
(a)
Problem 2b
Using the information in Figure 16-2, calculate the values of ∆H° for the following reactions:
(b)
Problem 2c
Using the information in Figure 16-2, calculate the values of ∆H° for the following reactions:
(c)
Problem 3
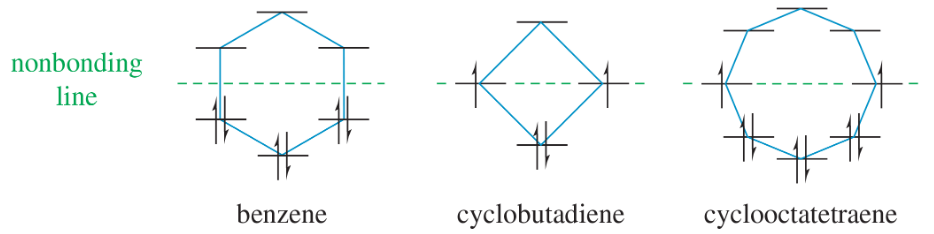
a. Draw the resonance forms of benzene, cyclobutadiene, and cyclooctatetraene, showing all the carbon and hydrogen atoms.
b. Assuming that these molecules are all planar, show how the p orbitals on the sp2 hybrid carbon atoms form continuous rings of overlapping orbitals above and below the plane of the carbon atoms.
Problem 4
Show the product of the Diels–Alder dimerization of cyclobutadiene. (This reaction is similar to the dimerization of cyclopentadiene, discussed in Section 15-11.)
Problem 5
Does the MO energy diagram of cyclooctatetraene (Figure 16-8) appear to be a particularly stable or unstable configuration? Explain.
Problem 6
Make a model of cyclooctatetraene in the tub conformation. Draw this conformation, and estimate the angle between the p orbitals of adjacent pi bonds.
Problem 7a,b
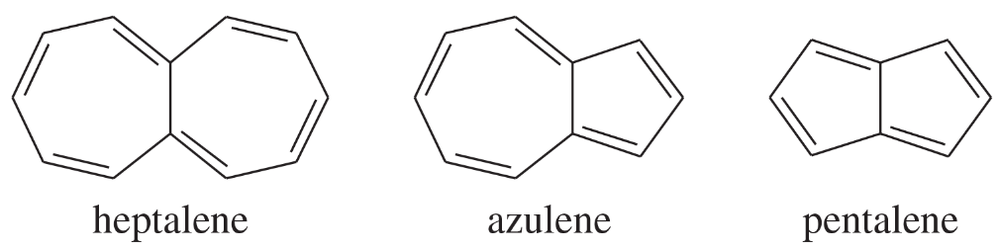
Classify the following compounds as aromatic, antiaromatic, or nonaromatic.
(a)
(b)
Problem 7c,d
Classify the following compounds as aromatic, antiaromatic, or nonaromatic.
(c)
(d)
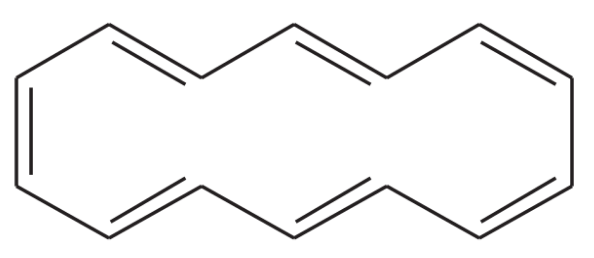
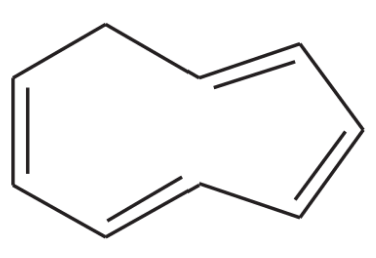
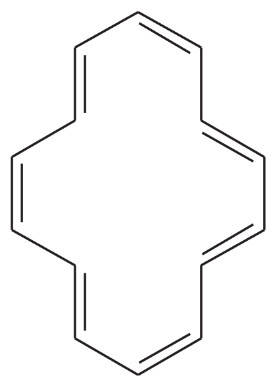
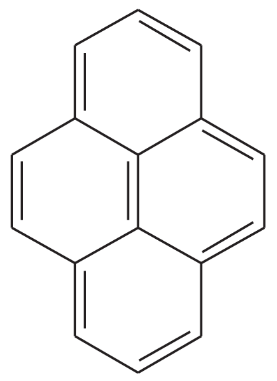
Problem 8
One of the following compounds is much more stable than the other two. Classify each as aromatic, antiaromatic, or nonaromatic.
Problem 9a,b
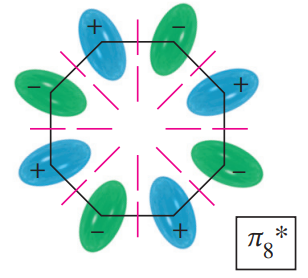
a. Use the polygon rule to draw an energy diagram (as in Figures 16-5 and 16-7) for the MOs of a planar cyclooctatetraenyl system.
b. Fill in the eight pi electrons for cyclooctatetraene. Is this electronic configuration aromatic or antiaromatic? Could the cyclooctatetraene system be aromatic if it gained or lost electrons?
Problem 9c
Drawpictorialrepresentations (as in Figures16-4 and 16-6) for the three bonding MOs and the two nonbonding MOs of cyclooctatetraene. The antibonding MOs are difficult to draw, except for the all-antibonding MO.
Problem 10
(a) Draw the molecular orbitals for the cyclopropenyl case.
(Because there are three p orbitals, there must be three MOs: one all-bonding MO and one degenerate pair of MOs.)
(b) Draw an energy diagram for the cyclopropenyl MOs. (The polygon rule is helpful.) Label each MO as bonding, nonbonding, or antibonding, and add the nonbonding line. Notice that it goes through the approximate average of the MOs.
(c) Add electrons to your energy diagram to show the configuration of the cyclopropenyl cation and the cyclopropenyl anion. Which is aromatic and which is antiaromatic?
Problem 11
Repeat Problem 16-10 for the cyclopentadienyl ions. Draw one all-bonding MO, then a pair of degenerate MOs, and then a final pair of degenerate MOs. Draw the energy diagram, fill in the electrons, and confirm the electronic configurations of the cyclopentadienyl cation and anion.