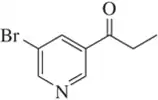
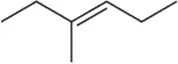
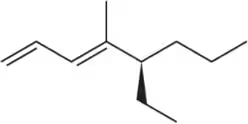
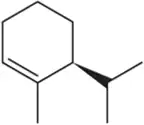
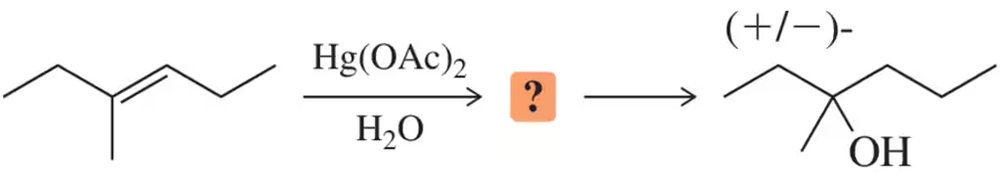Back
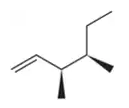
BackProblem 54a
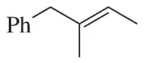
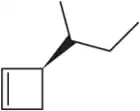
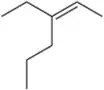
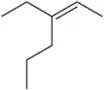
Predict the products you would get when the following alkenes undergo (i) hydroboration–oxidation (1. BH3 2. NaOH, H2O2 or (ii) oxymercuration–reduction [1. Hg(OAc)2, H2O 2. NaBH4].
(a)
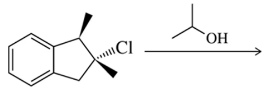
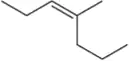
Problem 54b(ii)
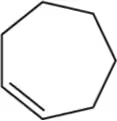
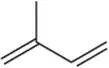
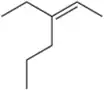
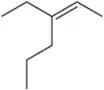
Predict the products you would get when the following alkenes undergo (ii) oxymercuration–reduction [1. Hg(OAc)2 , H2O 2. NaBH4 ].
(b)
Problem 55c
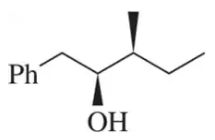
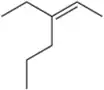
Suggest an alkene to undergo hydroboration–oxidation (1. BH3 2. NaOH, H2O2) to give exclusively the alcohols shown. Pay close attention to the relative (but not absolute) stereochemical outcome.
(c)
Problem 55d
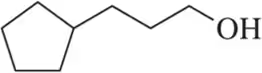
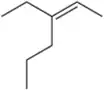
Suggest an alkene to undergo hydroboration–oxidation (1. BH3 2. NaOH, H2O2) to give exclusively the alcohols shown. Pay close attention to the relative (but not absolute) stereochemical outcome.
(d)
Problem 55e
Predict the major product(s) of the following elimination reactions, paying close attention to the stereochemical outcome of the reactions.
(e)
Problem 56
Unlike hydroboration–oxidation, the addition of H2O catalyzed by H3O+ is not stereospecific. Thinking carefully about the mechanism of the reaction, give two reasons why.
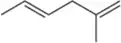
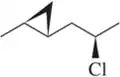
Problem 57
The first step of oxymercuration–reduction is stereospecific, and yet this fact wasn't emphasized in that discussion. Show the stereospecificity of the first step for the following alkene and then explain why the stereospecificity becomes unimportant after the second step.
Problem 58a
Calculate the index of hydrogen deficiency for the following molecular formulas and structures.
(a)
Problem 58b
Calculate the index of hydrogen deficiency for the following molecular formulas and structures.
(b)
Problem 58c
Calculate the index of hydrogen deficiency for the following molecular formulas and structures.
(c)
Problem 58f
Calculate the index of hydrogen deficiency for the following molecular formulas and structures.
(f) C6H8O2
Problem 59a
Using IUPAC rules, name the following molecules.
(a)
Problem 59c
Using IUPAC rules, name the following molecules.
(c)
Problem 59d
Using IUPAC rules, name the following molecules.
(d)
Problem 59e
Using IUPAC rules, name the following molecules.
(e)
Problem 59g
Using IUPAC rules, name the following molecules.
(g)
Problem 60b
Given the name, draw the structure of the following compounds.
(b) (4Z,8R)-8-bromo-5-methylnon-4-ene
Problem 60c
Given the name, draw the structure of the following compounds.
(c) (3S,6Z)-8-ethyl-3-iododeca-1,5-diene
Problem 60e
Given the name, draw the structure of the following compounds.
(e) (3R,5S)-5-chloro-3-isopropylcycloheptene
Problem 61b(iv)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iv) H2SO4 , H2O
(b)
Problem 61b(vi)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (vi) 1. BH3 2. H2O2, NaOH
(b)
Problem 61b(v)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (v) 1. Hg(OAc)2 , H2O 2. NaBH4
(b)
Problem 61b(i)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (i) HBr;
(b)
Problem 61b(iii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iii) HBr, H2O2
(b)
Problem 61b(ii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions:(ii) HCl;
(b)
Problem 61d
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (i) HBr; (ii) HCl; (iii) HBr, H2O2 (iv) H2SO4, H₂O (v) 1. Hg(OAc)2, H2O 2. NaBH4 ; (vi) 1. BH3 2. H2O2, NaOH
(d)
Problem 61g(ii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions:(ii) HCl
(g)
Problem 61g(vi)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (vi) 1. BH3 2. H2O2, NaOH
(g)
Problem 61g(iii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iii) HBr, H2O2
(g)
Problem 61g(v)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (v) 1. Hg(OAc)2 , H2O 2. NaBH4
(g)