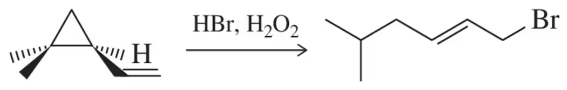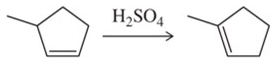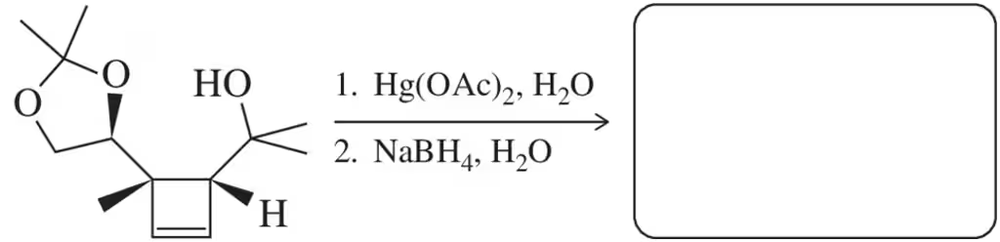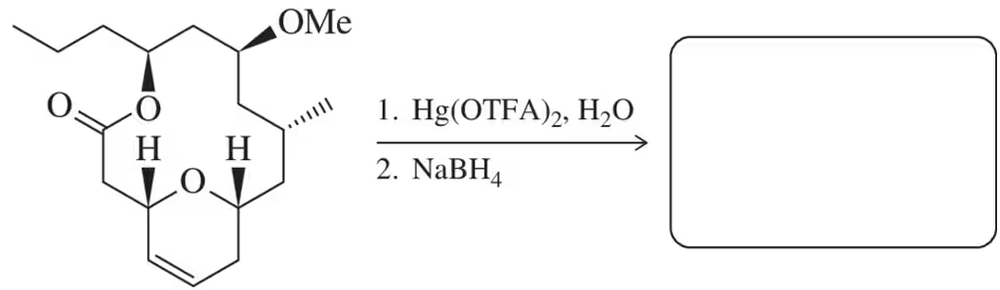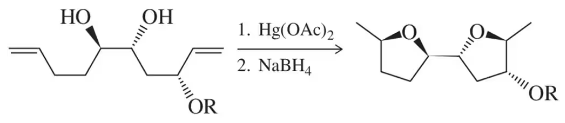Back
BackProblem 61g(v)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (v) 1. Hg(OAc)2 , H2O 2. NaBH4
(g)
Problem 61g(iv)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iv) H2SO4 , H2O
(g)
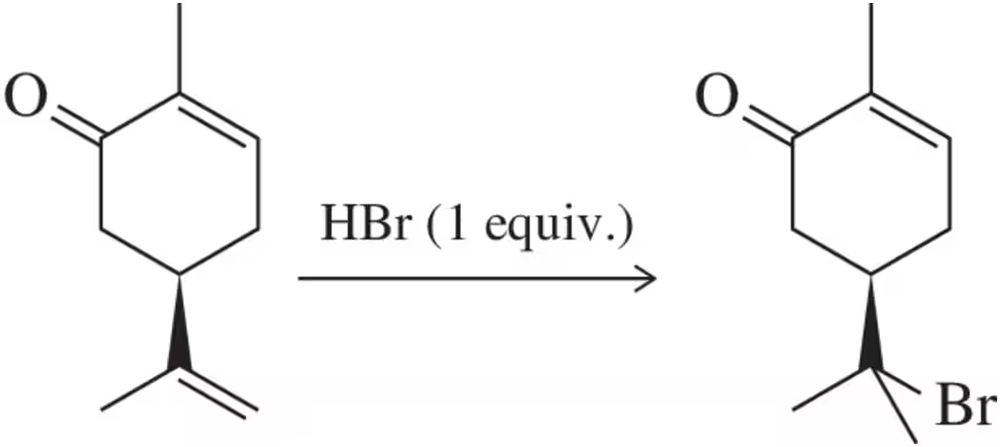
Problem 61k(i)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (i) HBr
(k)
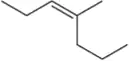
Problem 61k(v)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (v) 1. Hg(OAc)2 , H2O 2. NaBH4
(k)
Problem 61k(iv)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iv) H2SO4 , H2O
(k)
Problem 61k(ii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions:(ii) HCl
(k)
Problem 61k(vi)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (vi) 1. BH3 2. H2O2, NaOH
(k)
Problem 61k(iii)
Predict the product(s) that would result when the alkenes shown here are allowed to react under the following conditions: (iii) HBr, H2O2
(k)
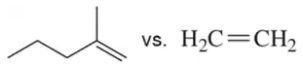
Problem 62b
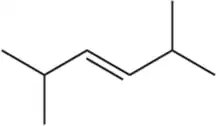
Which of the following alkenes would you expect to react fastest with HBr in each pair?
(b)
Problem 64
Which of the following cycloalkanes would you expect to produce the least heat upon combustion when measured per CH2? Explain your answer.
Problem 65
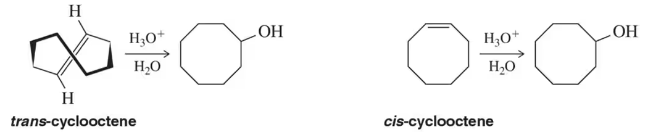
Cyclooctene is one of the smaller rings that can form a trans alkene. Would you expect cis-cyclooctene or trans-cyclooctene to react more quickly in an acid-catalyzed hydration reaction?
Problem 66b
How many stereoisomers are possible for the following alkenes?
(b)
Problem 66c
How many stereoisomers are possible for the following alkenes?
(c)
Problem 66d
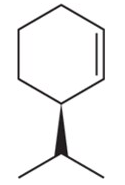
How many stereoisomers are possible for the following alkenes?
(d)
Problem 67
Because of the angle strain present in cyclopropanes, they tend to open up in the presence of nearby radicals. Show a mechanism for the following reaction that demonstrates this principle.
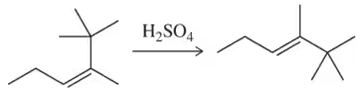
Problem 68a
When using sulfuric acid, but in the absence of other nucleophiles like water or bromide ion, less stable alkenes can be isomerized to their more stable isomer. Provide a mechanism for these acid-catalyzed isomerization reactions. [This is one illustration of the principle of microscopic reversibility.]
(a)
Problem 68b
When using sulfuric acid, but in the absence of other nucleophiles like water or bromide ion, less stable alkenes can be isomerized to their more stable isomer. Provide a mechanism for these acid-catalyzed isomerization reactions. [This is one illustration of the principle of microscopic reversibility.]
(b)
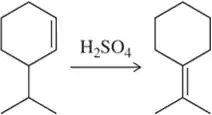
Problem 68c
When using sulfuric acid, but in the absence of other nucleophiles like water or bromide ion, less stable alkenes can be isomerized to their more stable isomer. Provide a mechanism for these acid-catalyzed isomerization reactions. [This is one illustration of the principle of microscopic reversibility.]
(c)
Problem 70
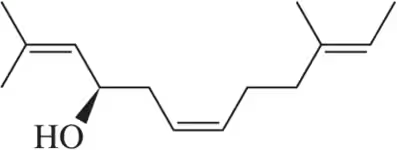
If you react carvone with a single equivalent of HBr, only one product is produced, even though carvone has two carbon–carbon double bonds. Explain this observation.
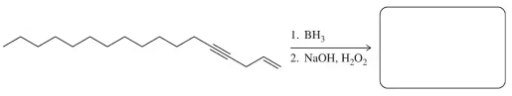
Problem 71b
Predict the product of each of the following hydroboration–oxidation or oxymercuration–reduction reactions used in the modern synthetic organic chemistry literature (modified to use reagents we are used to seeing).
(b) A two-step hydroboration–oxidation was used to prepare a silanediol peptidomimetic as a serine protease inhibitor (Org. Lett. 2012, 14, 4422–4425).
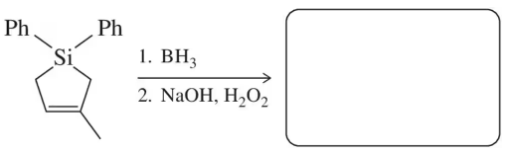
Problem 71c
Predict the product of each of the following hydroboration–oxidation or oxymercuration–reduction reactions used in the modern synthetic organic chemistry literature (modified to use reagents we are used to seeing).
(c) A similar sequence was featured in the synthesis of muricadienin, a proposed precursor in the biosynthesis of solamin (Org. Lett. 2014, 16, 5886–5889).
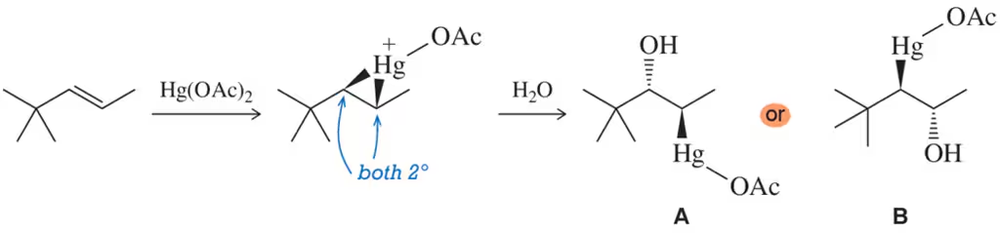
Problem 73
A common situation occurs when both carbons of the mercurinium ion are secondary. In a situation like the one shown, to which carbon would you expect water to add? That is, would you expect to produce A or B? Explain your answer.
Problem 74b
In light of your answer to Assessment 8.73, predict the product of the following oxymercuration–reduction reactions, each of which results in a single product. [Don't worry about the absolute stereochemistry, though these reactions are also stereoselective.]
(b) This reaction sequence was used in the synthesis of (+) -lineatin, a monoterpene aggregation pheromone from the female ambrosia beetle Trypodendron lineatum (Org. Lett. 2004, 6, 1449–1452).
Problem 74c
In light of your answer to Assessment 8.73, predict the product of the following oxymercuration–reduction reactions, each of which results in a single product. [Don't worry about the absolute stereochemistry, though these reactions are also stereoselective.]
(c) Oxymercuration–reduction was used in the stereoselective synthesis of the macrolactone core of neopeltolide, a marine macrolide isolated from a Caribbean sponge that has potent anticancer activity (Org. Lett. 2012, 14, 2346–2349).
Problem 75
The formation of five-membered ring ethers is an important goal in synthetic organic chemistry because tetrahydrofurans are contained within a number of antitumor natural products. Toward that end, a one-pot synthesis of a bis-THF containing compound was developed (Eur. J. Org. Chem. 2010, 6263–6268). Suggest a mechanism for this transformation.
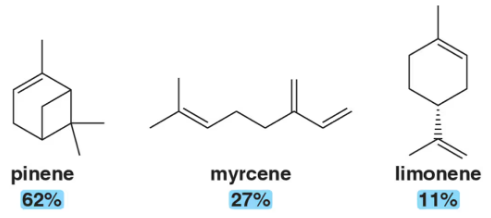
Problem 76a
North American termite soldiers, when encountering enemy insects, contract their mandibular muscles, expelling a mixture of chemicals that essentially trap their enemies in a glue-like substance. This weapon, built into the face of the termite, is called the fontanellar gun. It releases a mixture of pinene (62%), myrcene (27%), and limonene (11%). pinene myrcene limonene 62% 27% 11%
(a) Identify the isoprene units in pinene, myrcene, and limonene.
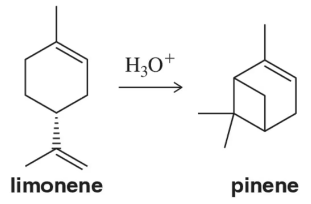
Problem 76b
North American termite soldiers, when encountering enemy insects, contract their mandibular muscles, expelling a mixture of chemicals that essentially trap their enemies in a glue-like substance. This weapon, built into the face of the termite, is called the fontanellar gun. It releases a mixture of pinene (62%), myrcene (27%), and limonene (11%).
(b) Suggest an acid-catalyzed mechanism by which pinene could be produced from limonene.
Problem 77a
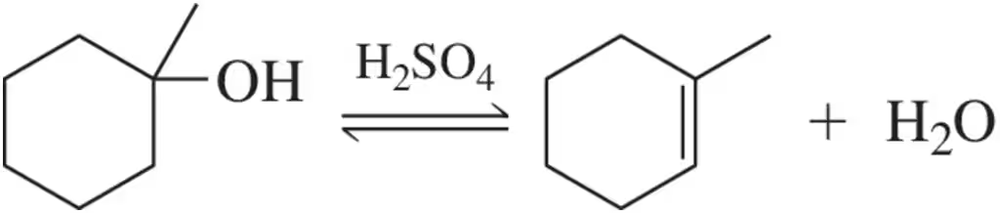
The acid-catalyzed hydration we learned here in Chapter 8 is reversible.
(a) Propose a mechanism for the formation of an alkene from an alcohol.
Problem 77c
The acid-catalyzed hydration we learned here in Chapter 8 is reversible:
(c) Which side of the reaction would be favored by running the reaction at high temperatures?
Problem 77d
The acid-catalyzed hydration we learned here in Chapter 8 is reversible:
(d) How might you shift the equilibrium to the right?