Back
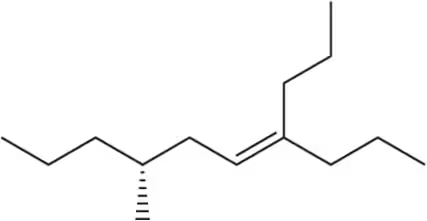
BackProblem 6a
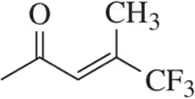
Given the structure, calculate the index of hydrogen deficiency (degrees of unsaturation) of the following molecules.
(a)
Problem 7a
What is the index of hydrogen deficiency for each of the following molecular formulas?
(a) C6H12O6
Problem 7b
What is the index of hydrogen deficiency for each of the following molecular formulas?
(b) C12H11NO3
Problem 7c
What is the index of hydrogen deficiency for each of the following molecular formulas?
(c) C8H14Cl2
Problem 7d
What is the index of hydrogen deficiency for each of the following molecular formulas?
(d) C9H12N2O2
Problem 7e
What is the index of hydrogen deficiency for each of the following molecular formulas?
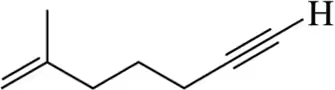
(e) C6H12O2
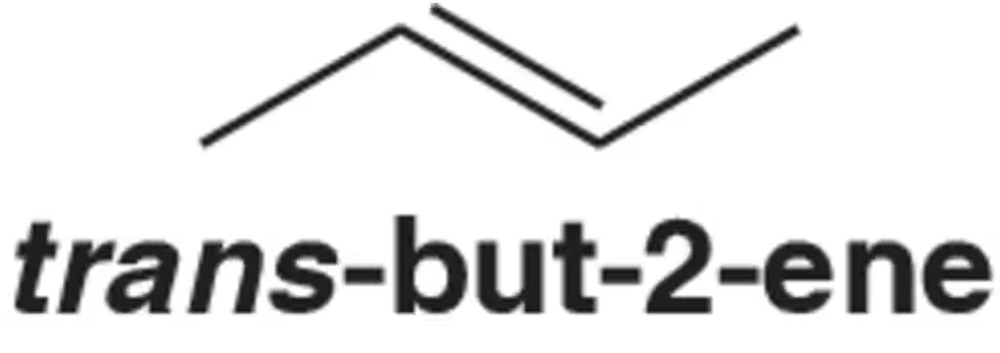
Problem 8
Draw the molecular orbital picture of trans-but-2-ene.
Problem 8.39d
Provide the expected product for the reaction of each of the following alkenes with H₂SO₄ and H₂O.
(d)
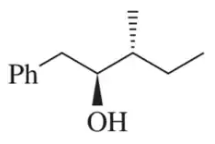
Problem 8.55b
Suggest an alkene to undergo hydroboration–oxidation (1. BH3 2. NaOH, H2O2) to give exclusively the alcohols shown. Pay close attention to the relative (but not absolute) stereochemical outcome.
(b)
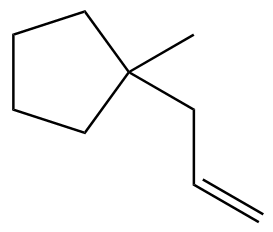
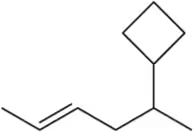
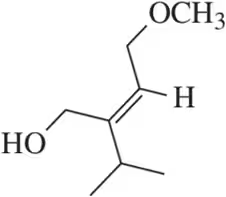
Problem 9a
Provide the IUPAC names for the following alkenes.
(a)
Problem 10a
Given the following IUPAC names, draw the corresponding structures.
(a) (R)-3-isopropyl-6-methylnon-1-ene
Problem 10c
Given the following IUPAC names, draw the corresponding structures.
(c) (S)-3-fluoropent-1-ene
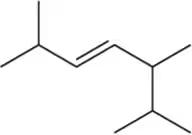
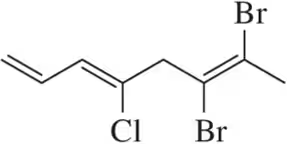
Problem 11a
Name the following alkenes, being sure to specify whether they are cis or trans.
(a)
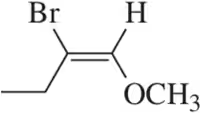
Problem 11c
Name the following alkenes, being sure to specify whether they are cis or trans.
(c)
Problem 12a
Which of the following alkenes are E and which are Z?
(a)
Problem 12b
Which of the following alkenes are E and which are Z?
(b)
Problem 12c
Which of the following alkenes are E and which are Z?
(c)
Problem 13a
Given the name, draw the structure of the following alkenes.
(a) (E)-4-ethyl-5-methyloct-3-ene
Problem 13b
Given the name, draw the structure of the following alkenes.
(b) ((Z)-1-cyclohexyl-2-methylhept-2-ene
Problem 13c
Given the name, draw the structure of the following alkenes.
(c) (Z)-3-isopropylhept-3-ene
Problem 15b
Name the following alkenes.
(b)
Problem 16
How many stereoisomers are possible (a) for 4-methylnona-2,7-diene? (b) For 6-chloronona-2,4,7-triene?
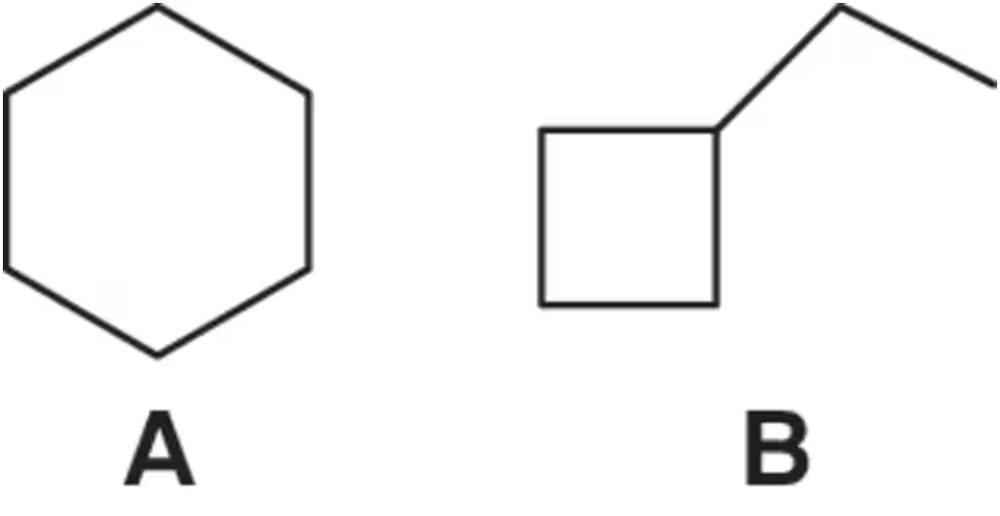
Problem 17
Which constitutional isomer, A or B, would you expect to have the highest heat of combustion (∆Hcombustion)?
Problem 18a
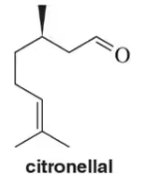
For the following terpenes, identify the isoprene units. In cross-linked or ring-containing terpenes, linkages can be formed between more than just C1 and C4 of isoprene.
(a)
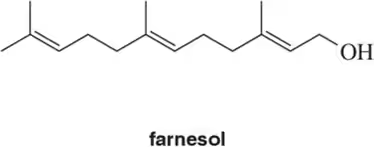
Problem 18b
For the following terpenes, identify the isoprene units. In cross-linked or ring-containing terpenes, linkages can be formed between more than just C1 and C4 of isoprene
(b)
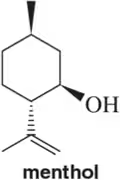
Problem 18c
For the following terpenes, identify the isoprene units. In cross-linked or ring-containing terpenes, linkages can be formed between more than just C1 and C4 of isoprene
(c)
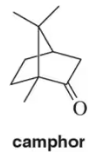
Problem 18d
For the following terpenes, identify the isoprene units. In cross-linked or ring-containing terpenes, linkages can be formed between more than just C1 and C4 of isoprene.
(d)
Problem 19c
Predict the product(s) of each of the following reactions. If you expect a racemic mixture, draw both enantiomers.
(c)
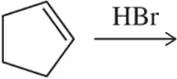
Problem 20a(a)
The following reaction steps are shown using conventional electron pushing. (a) Draw the second product whose formation would have been rationalized with this same arrow.
(a)
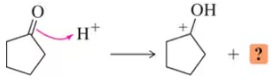
Problem 20a(b)
The following reaction steps are shown using conventional electron pushing. (b) Use the bouncing arrow formalism to illustrate the formation of only the product shown.
(a)