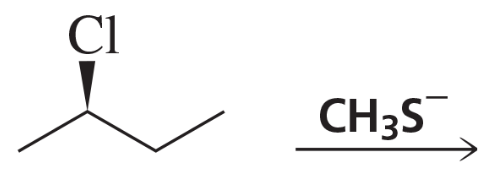
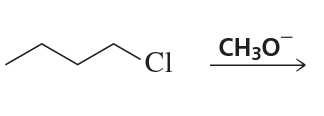
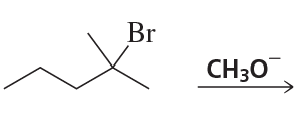
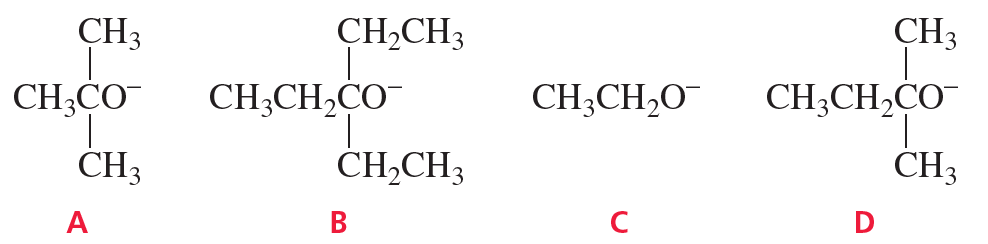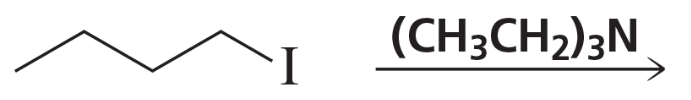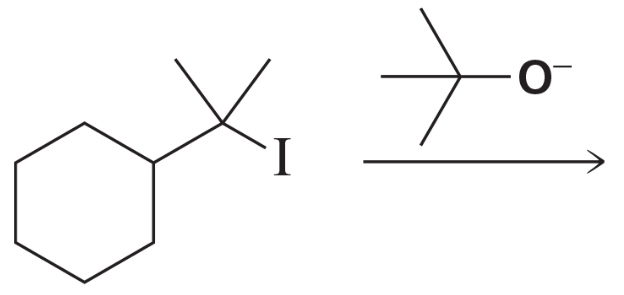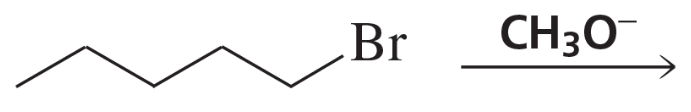Back
BackProblem 93c
Indicate which species in each pair gives a higher substitution-product-to-elimination-product ratio when it reacts with isopropyl bromide:
c. Cl− or Br−
Problem 94
Rank the following from most reactive to least reactive in an E2 reaction:
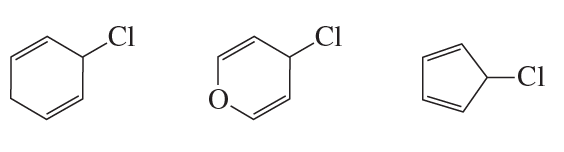
Problem 95
Rank the following from most reactive to least reactive in an SN1 reaction:
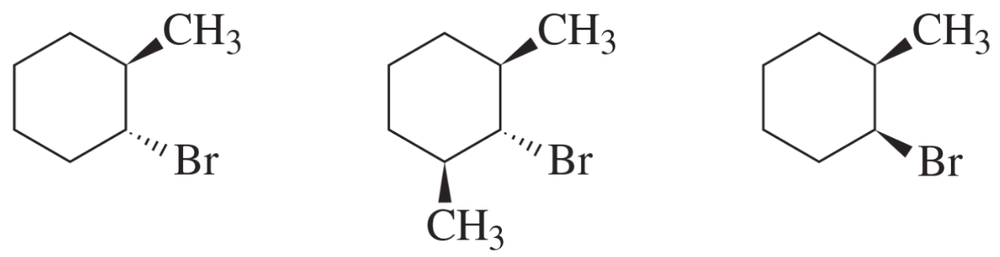
Problem 96a
For each of the following alkyl halides, indicate the stereoisomer that would be obtained in greatest yield in an E2 reaction.
a. 3-bromo-2,2,3-trimethylpentane
Problem 96b
For each of the following alkyl halides, indicate the stereoisomer that would be obtained in greatest yield in an E2 reaction.
b. 4-bromo-2,2,3,3-tetramethylpentane
Problem 96c,d
For each of the following alkyl halides, indicate the stereoisomer that would be obtained in greatest yield in an E2 reaction.
c. 3-bromo-2,3-dimethylpentane
d. 3-bromo-3,4-dimethylhexane
Problem 96d
For each of the following alkyl halides, indicate the stereoisomer that would be obtained in greatest yield in an E2 reaction.
d. 3-bromo-3,4-dimethylhexane
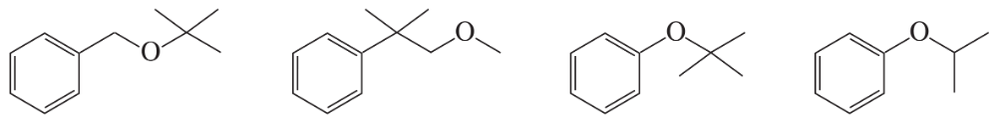
Problem 97
Which of following ethers cannot be made by a Williamson ether synthesis?
Problem 98
When 2-bromo-2,3-dimethylbutane reacts with a strong base, two alkenes (2,3-dimethyl-1-butene and 2,3-dimethyl-2-butene) are formed.
a. Which of the bases (A, B, C, or D) would form the highest percentage of the 1-alkene?
b. Which would give the highest percentage of the 2-alkene?
Problem 99a,b
What are the products of the following reactions?
a.
b.
Problem 99e
What are the products of the following reactions?
e.
Problem 99f
What are the products of the following reactions?
f.
Problem 99g,h
What are the products of the following reactions?
g.
h.
Problem 100a,b
a. Draw the structures of the products obtained from the reaction of each enantiomer of cis-1-chloro-2-isopropylcyclopentane with sodium methoxide in methanol.
b. Are all the products optically active?
Problem 101
When the following compound undergoes solvolysis in ethanol, three products are obtained. Propose a mechanism to account for the formation of these products.
Problem 102
cis-1-Bromo-4-tert-butylcyclohexane and trans-1-bromo-4-tert-butylcyclohexane both react with sodium ethoxide in ethanol to form 4-tert-butylcyclohexene. Explain why the cis isomer reacts much more rapidly than the trans isomer.
Problem 103a,b
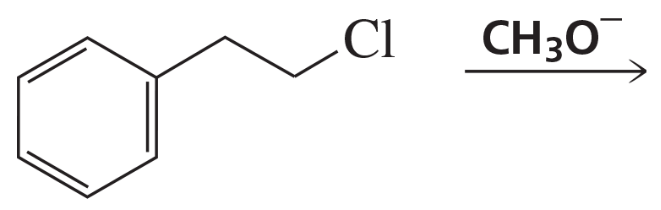
Draw the substitution and elimination products.
a.
b.
Problem 104
tert-Butyl chloride undergoes solvolysis in both acetic acid and formic acid. Solvolysis occurs 5000 times faster in one of these two solvents than in the other. In which solvent is solvolysis faster? Explain your answer. (Hint: Formic acid is more polar than acetic acid.)
Problem 105
What substitution products are obtained when each of the following compounds is added to a solution of sodium acetate in acetic acid?
a. 2-chloro-2-methyl-3-hexene
b. 3-bromo-1-methylcyclohexene
Problem 106a
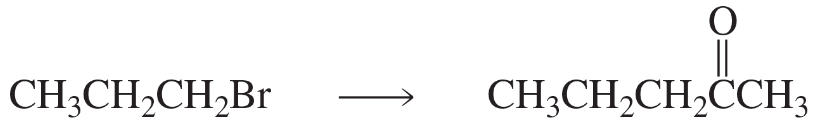
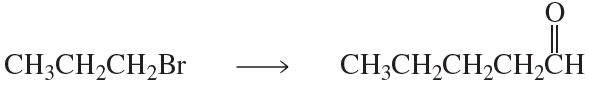
Show how each of the following compounds can be synthesized from the given starting materials:
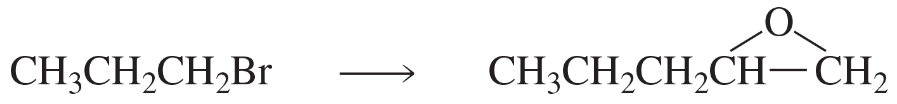
a. CH3CH2CH2Br→CH3CH2CH2CH2CH3
Problem 106b
Show how each of the following compounds can be synthesized from the given starting materials:
b.
Problem 106c
Show how each of the following compounds can be synthesized from the given starting materials:
c.
Problem 106d
Show how each of the following compounds can be synthesized from the given starting materials:
d.
Problem 107
In which solvent—ethanol or diethyl ether—would the equilibrium for the following SN2 reaction lie farther to the right?
Problem 108a
The rate of the reaction of methyl iodide with quinuclidine was measured in nitrobenzene, and then the rate of the reaction of methyl iodide with triethylamine was measured in the same solvent. The concentration of the reagents was the same in both experiments.
a.Which reaction had the larger rate constant?
Problem 108b
The rate of the reaction of methyl iodide with quinuclidine was measured in nitrobenzene, and then the rate of the reaction of methyl iodide with triethylamine was measured in the same solvent. The concentration of the reagents was the same in both experiments.
b. The same experiment was done using isopropyl iodide instead of methyl iodide. Which reaction had the larger rate constant?
Problem 108c
The rate of the reaction of methyl iodide with quinuclidine was measured in nitrobenzene, and then the rate of the reaction of methyl iodide with triethylamine was measured in the same solvent. The concentration of the reagents was the same in both experiments.
c. Which alkyl halide has the larger kquinuclidine/ktriethylamine ratio?
Problem 109
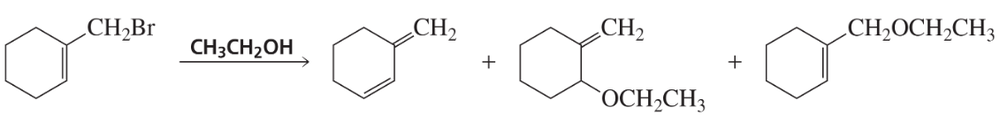
Two bromoethers are obtained from the reaction of the following alkyl dihalide with methanol. Draw the structures of the ethers.

Problem 110a,b
Draw the substitution products for each of the following SN2 reactions. If the products can exist as stereoisomers, show which stereoisomers are formed:
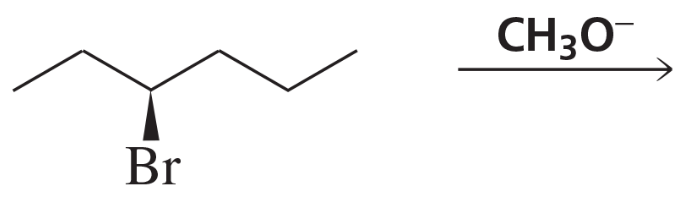
a. (3S,4S)-3-bromo-4-methylhexane + CH3O-
b. (3S,4R)-3-bromo-4-methylhexane + CH3O-
Problem 110c,d
Draw the substitution products for each of the following SN2 reactions. If the products can exist as stereoisomers, show which stereoisomers are formed:
c. (3R,4R)-3-bromo-4-methylhexane + CH3O-
d. (3R,4S)-3-bromo-4-methylhexane + CH3O-