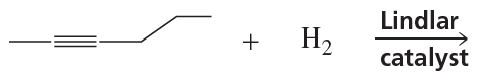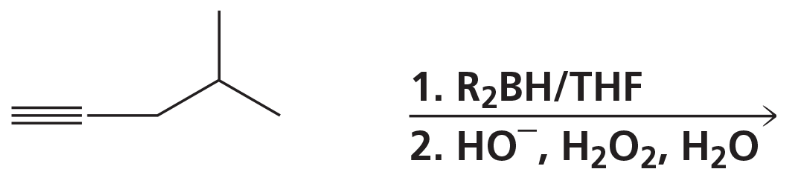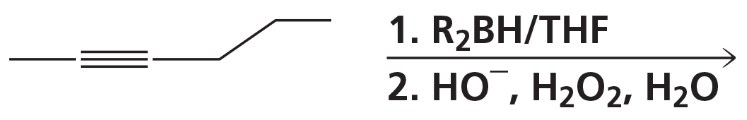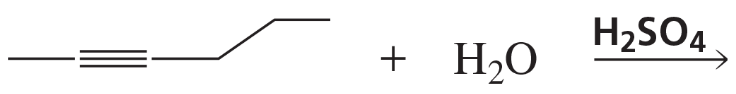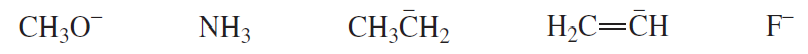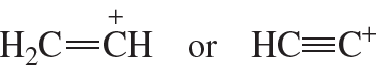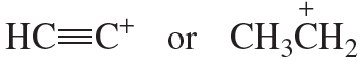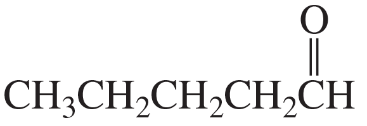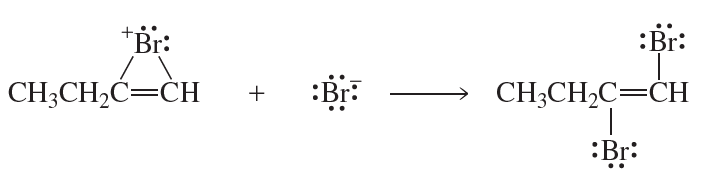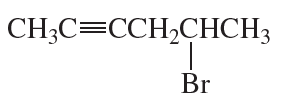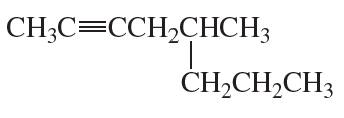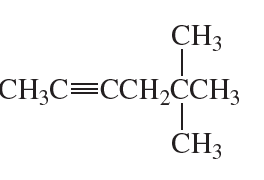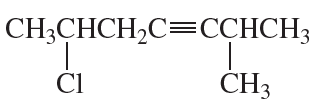Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.7 - The Reactions of Alkynes An Introduction to Multistep Synthesis
Ch.7 - The Reactions of Alkynes An Introduction to Multistep SynthesisProblem 20b
What are products of the following reactions?
b.
Problem 20c
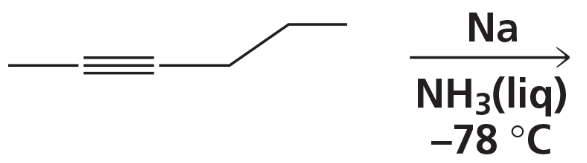
What are products of the following reactions?
c.
Problem 20d
What are products of the following reactions?
d.
Problem 20e
What are products of the following reactions?
e.
Problem 20f
What are products of the following reactions?
f.
Problem 21
Which of the following bases can remove a proton from a terminal alkyne in a reaction that favors products?
Problem 22
Explain why an amide ion cannot be used to form a carbanion from an alkane in a reaction that favors products.
Problem 23
Any base whose conjugate acid has a pKa greater than ______ can remove a proton from a terminal alkyne to form an acetylide ion (in a reaction that favors products).
Problem 26a
Which carbocation is more stable?
a.
Problem 26b
Which carbocation is more stable?
b.
Problem 28b
How could the following compounds be synthesized from acetylene?
b.
Problem 28c
How could the following compounds be synthesized from acetylene?
c. CH3CH═CH2
Problem 28d
How could the following compounds be synthesized from acetylene?
d.
Problem 28e
How could the following compounds be synthesized from acetylene?
e.
Problem 29c
What is the major product obtained from the reaction of each of the following compounds with excess HCl?
c. CH3CH2C☰CCH2CH2CH3
Problem 30a,b
Draw a condensed structure for each of the following:
a. 2-hexyne
b. 5-ethyl-3-octyne
Problem 30e,f
Draw a condensed structure for each of the following:
e. methoxyethyne
f. sec-butyl-tert-butylacetylene
Problem 30g,h
Draw a condensed structure for each of the following:
g. 1-bromo-1-pentyne
h. 5-methyl-2-cyclohexenol
Problem 30i,j
Draw a condensed structure for each of the following:
i. diethylacetylene
j. di-tert-butylacetylene
Problem 30k,l
Draw a condensed structure for each of the following:
k. cyclopentylacetylene
l. 5,6-dimethyl-2-heptyne
Problem 31a,b
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did she name correctly? Correct those that are misnamed.
a. 4-ethyl-2-pentyne
b. 1-bromo-4-heptyne
Problem 31c
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did she name correctly? Correct those that are misnamed.
c. 2-methyl-3-hexyne d. 3-pentyne
Problem 32a
Identify the electrophile and the nucleophile in each of the following reaction steps. Then draw curved arrows to illustrate the bond-making and bond-breaking processes.
a.
Problem 33a
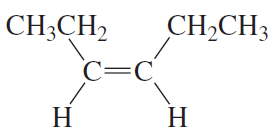
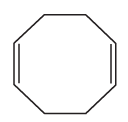
What is each compound's systematic name?
a.
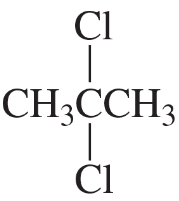
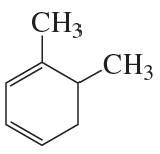
Problem 33b
What is each compound's systematic name?
b.
Problem 33c
What is each compound's systematic name?
c.
Problem 33d
What is each compound's systematic name?
d.
Problem 33e,f
What is each compound's systematic name?
e.
f.
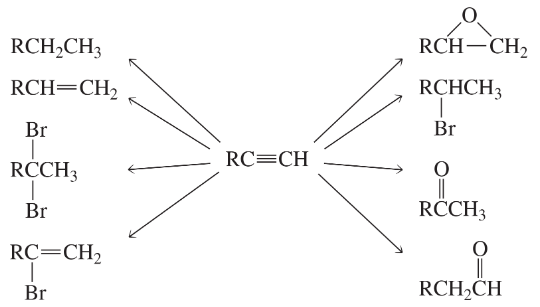
Problem 34a
What reagents should be used to carry out the following syntheses?
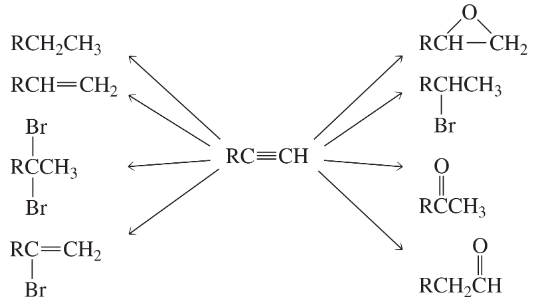
Problem 34b,c
What reagents should be used to carry out the following syntheses?