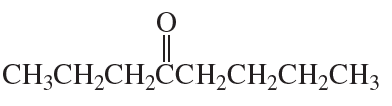Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.7 - The Reactions of Alkynes An Introduction to Multistep Synthesis
Ch.7 - The Reactions of Alkynes An Introduction to Multistep SynthesisProblem 34d
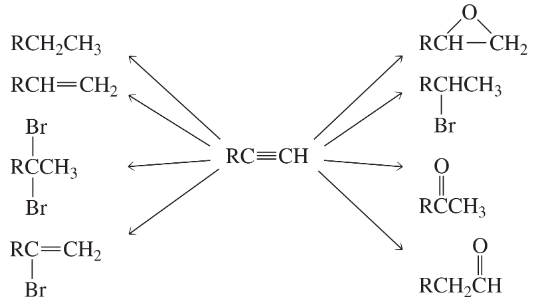
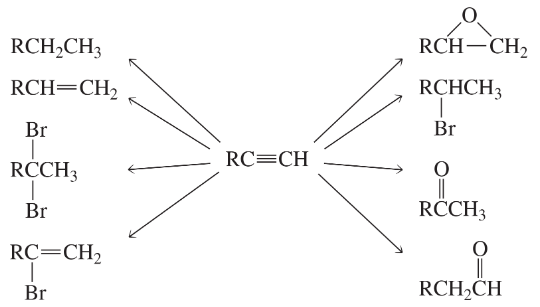
What reagents should be used to carry out the following syntheses?
Problem 34e
What reagents should be used to carry out the following syntheses?
Problem 34f
What reagents should be used to carry out the following syntheses?
Problem 34g
What reagents should be used to carry out the following syntheses?
Problem 35a
Draw the structures and give the common and systematic names for alkynes with molecular formula C7H12. Ignore stereosiomers. (Hint: There are 14.)
Problem 35b
How many would there be if stereoisomers are included?
Problem 36
Draw the mechanism for the following reaction:
Problem 37b
How can the following compounds be synthesized, starting with a hydrocarbon that has the same number of carbons as the desired product?
b. CH3CH2CH2CH2OH
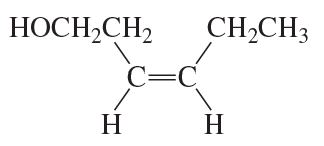
Problem 37c
How can the following compounds be synthesized, starting with a hydrocarbon that has the same number of carbons as the desired product?
c.
Problem 38a
What reagents would you use for the following syntheses?
a. (Z)-3-hexene from 3-hexyne
Problem 38b
What reagents would you use for the following syntheses?
b. (E)-3-hexene from 3-hexyne
Problem 38c
What reagents would you use for the following syntheses?
c. hexane from 3-hexyne
Problem 39c
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
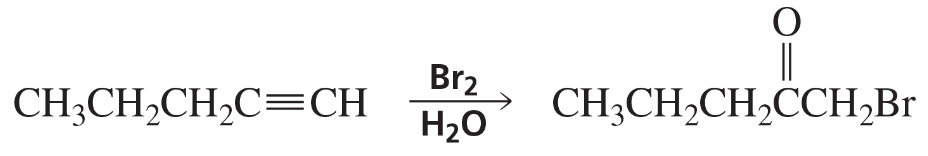
c. Br2 (1 mol)/CH2Cl2
Problem 39d
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
d. Br2 (2 mol)/CH2Cl2
Problem 39e,f
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
e. aqueous H2SO4, HgSO4
f. R2BH in THF followed by H2O2/HO− /H2O
Problem 39g,h
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
g. excess H2, Pd/C
h. H2/Lindlar catalyst
Problem 39i
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
i. sodium amide
Problem 39j
What is the major product of the reaction of 1 mol of propyne with each of the following reagents?
j. the product of part i followed by 1-chloropropane
Problem 40a,b
Answer Problem 39, parts a–h, using 2-butyne as the starting material instead of propyne.
a. HBr (1 mol)
b. HBr (2 mol)
Problem 40c
Answer Problem 39 , parts a–h, using 2-butyne as the starting material instead of propyne.
c. Br2 (1 mol)/CH2Cl2
Problem 40d
Answer Problem 39 , parts a–h, using 2-butyne as the starting material instead of propyne.
d. Br2 (2 mol)/CH2Cl2
Problem 40e
Answer Problem 39, parts a–h, using 2-butyne as the starting material instead of propyne.
e. aqueous H2SO4, HgSO4
Problem 40f
Answer Problem 39, parts a–h, using 2-butyne as the starting material instead of propyne.
f. R2BH in THF followed by H2O2/HO− /H2O
Problem 40g
Answer Problem 39, parts a–h, using 2-butyne as the starting material instead of propyne.
g. excess H2, Pd/C
Problem 40h
Answer Problem 39, parts a–h, using 2-butyne as the starting material instead of propyne.
h. H2/Lindlar catalyst
Problem 41a
What is each compound's systematic name?
a. CH3C☰CCH2CH2CH2CH═CH2
Problem 41b
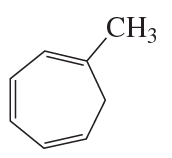
What is each compound's systematic name?
b.
Problem 41e,f
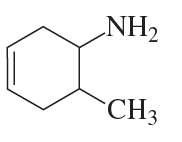
What is each compound's systematic name?
e.
f.
Problem 42
What is the molecular formula of a hydrocarbon that has 1 triple bond, 2 double bonds, 1 ring, and 32 carbons?
Problem 43(2)
a. Starting with 3-methyl-1-butyne, how can you prepare the following alcohols?
2. 3-methyl-1-butanol
b. In each case, a second alcohol would also be obtained. What alcohol would it be?