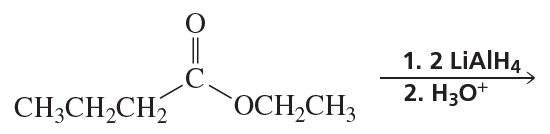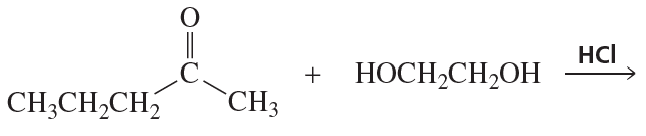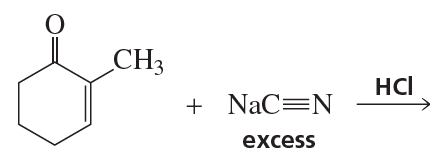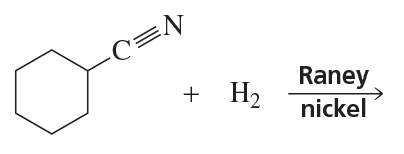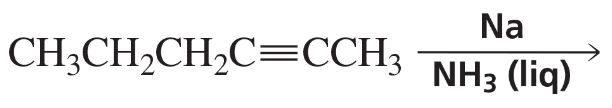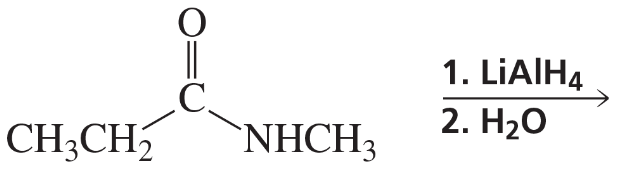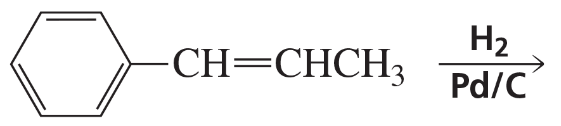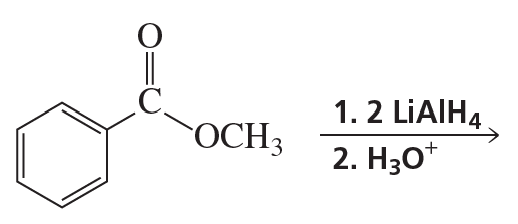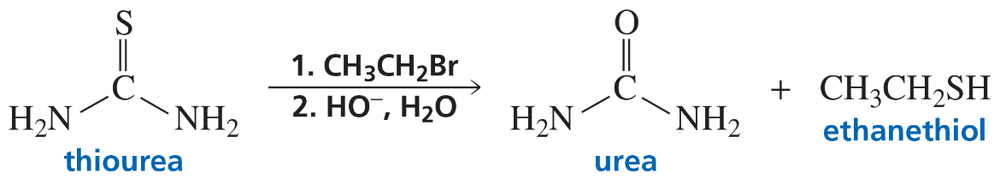Back
Back Bruice 8th Edition
Bruice 8th Edition Ch. 16 - Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid Derivatives
Ch. 16 - Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid DerivativesProblem 54e
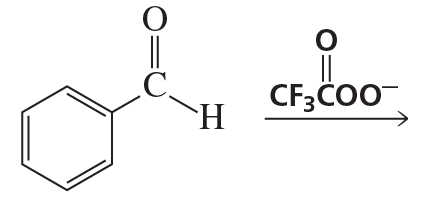
What are the products of the following reactions?
e.
Problem 54f
What are the products of the following reactions?
f.
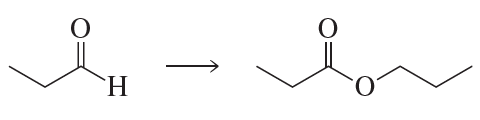
Problem 54g
What are the products of the following reactions?
g.
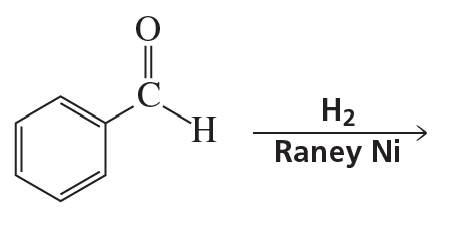
Problem 54h
What are the products of the following reactions?
h.
Problem 55
Rank the following compounds from most reactive to least reactive toward nucleophilic addition:
Problem 56
Draw the structure of two esters that will be reduced to propanol and butanol by LiAlH4 (followed by addition of aqueous acid).
Problem 57a(1)
Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(3)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(4)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(5)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(7)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(8)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57a(10)
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 57b
Which of the reactions cannot be used for the synthesis of isobutyl alcohol?
Problem 58a,b,c
Draw the products of the following reactions. Indicate whether each reaction is an oxidation or a reduction.
a.
b.
c.
Problem 58d
Draw the products of the following reactions. Indicate whether each reaction is an oxidation or a reduction.
d.
Problem 58e
Draw the products of the following reactions. Indicate whether each reaction is an oxidation or a reduction.
e.
Problem 58f
Draw the products of the following reactions. Indicate whether each reaction is an oxidation or a reduction.
f.
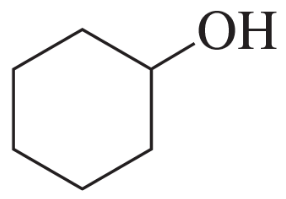
Problem 60a,b
Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:
a.
b.
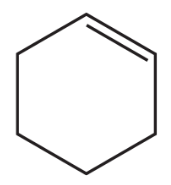
Problem 60c
Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:
c.
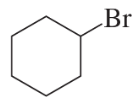
Problem 60d
Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:
d.
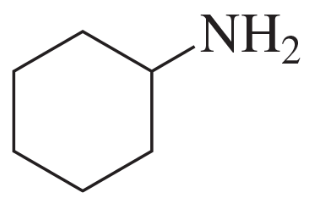
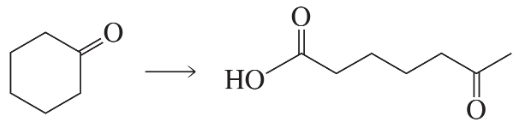
Problem 60e
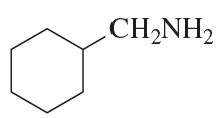
Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:
e.
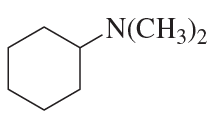
Problem 60f
Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:
f.
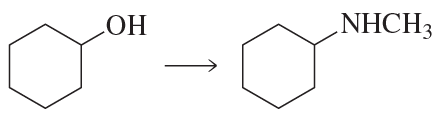
Problem 62a
Show how each of the following compounds can be prepared, using the given starting material:
a.
Problem 62c
Show how each of the following compounds can be prepared, using the given starting material:
c.
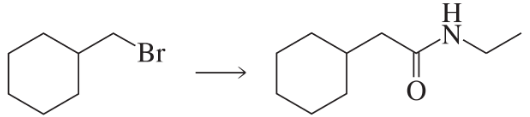
Problem 62e
Show how each of the following compounds can be prepared, using the given starting material:
e.
Problem 62f
Show how each of the following compounds can be prepared, using the given starting material:
f.
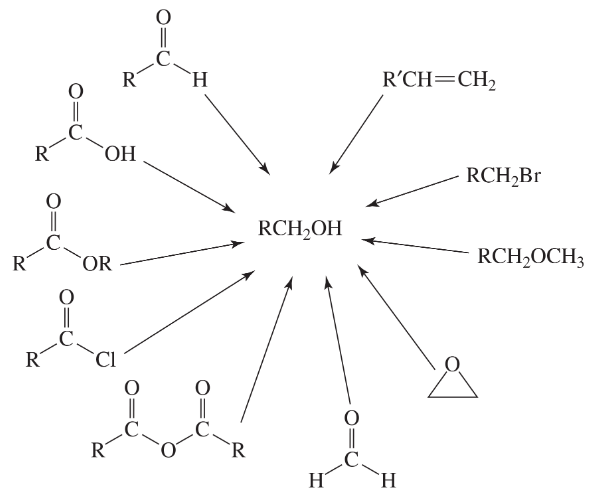
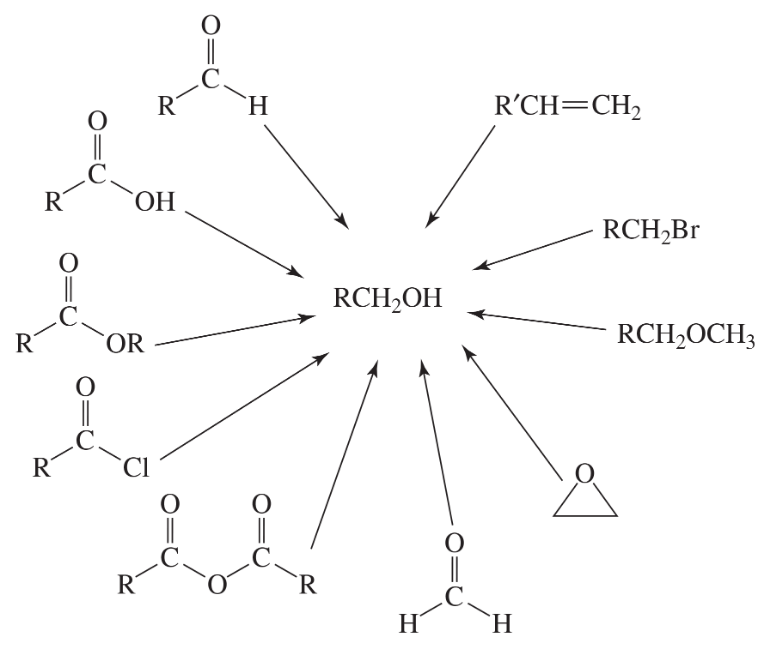
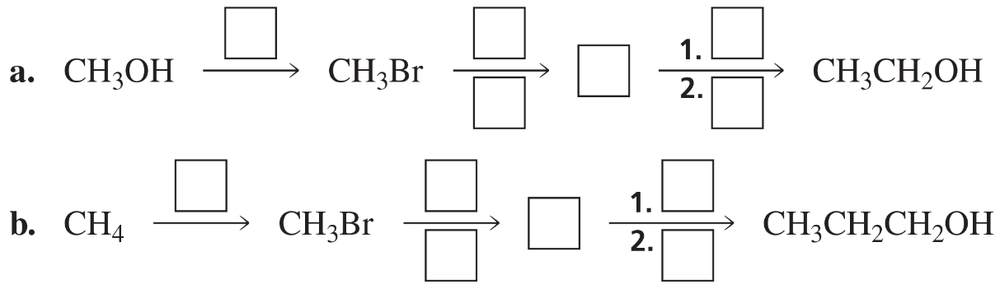
Problem 63
Fill in the boxes:
Problem 64a
Thiols can be prepared from the reaction of thiourea with an alkyl halide, followed by hydroxide-ion-promoted hydrolysis.
a. Propose a mechanism for the reaction.
Problem 64b
Thiols can be prepared from the reaction of thiourea with an alkyl halide, followed by hydroxide-ion-promoted hydrolysis.
b. What thiol will be formed if the alkyl halide employed is pentyl bromide?