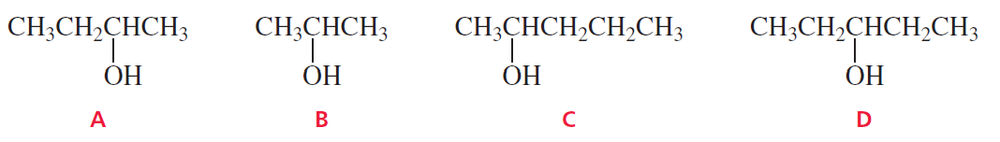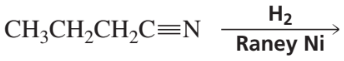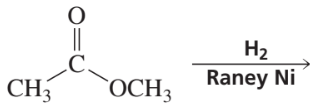Back
Back Bruice 8th Edition
Bruice 8th Edition Ch. 16 - Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid Derivatives
Ch. 16 - Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid DerivativesProblem 1a,b,c
Give two names for each of the following:
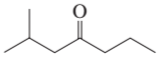
a.
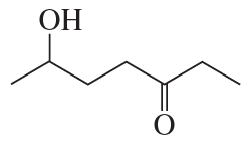
b.
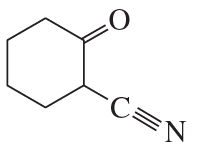
c.
Problem 2
Why are numbers not used to designate the position of the functional group in propanone and butanedione?
Problem 3a
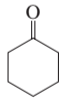
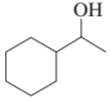
Name the following:
a.
Problem 3b
Name the following:
b.
Problem 3c
Name the following:
c.
Problem 5
What products are formed when the following compounds react with CH3MgBr, followed by the addition of dilute acid? Disregard stereoisomers.
a.
b.
c.
Problem 7a
How many stereoisomers are obtained from the reaction of 2-pentanone with ethylmagnesium bromide followed by the addition of dilute acid?
Problem 9
Which of the following secondary alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent?
Problem 11
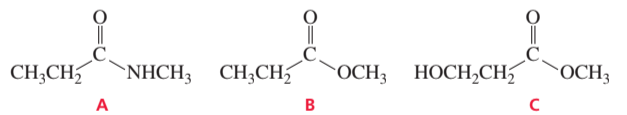
Which of the following compounds does not form an alcohol when it reacts with excess Grignard reagent?
Problem 12
Show how the following compounds can be synthesized from cyclohexanol.
a.
b.
c.
Problem 13a(1)
Show how the following compounds can be prepared, using ethyne as one of the starting materials:
1. 1-pentyn-3-ol
Problem 13a(2)
Show how the following compounds can be prepared, using ethyne as one of the starting materials:
2. 1-phenyl-2-butyn-1-ol
Problem 13a(3)
Show how the following compounds can be prepared, using ethyne as one of the starting materials:
3. 2-methyl-3-hexyn-2-ol
Problem 13b
Explain why ethyne should be alkylated before, rather than after, nucleophilic addition.
Problem 14
What is the product of the reaction of an ester with excess acetylide ion followed by the addition of pyridinium chloride?
- Can a cyanohydrin be prepared by treating a ketone with sodium cyanide?
Problem 16
Problem 20a,b
What alcohols are obtained from the reduction of the following compounds with sodium borohydride?
a. 2-methylpropanal
b. cyclohexanone
Problem 20c,d
What alcohols are obtained from the reduction of the following compounds with sodium borohydride?
c. 4-tert-butylcyclohexanone
d. acetophenone
Problem 21a,b
What products are obtained from the reaction of the following compounds with LiAlH4 followed by treatment with dilute acid?
a. ethyl butanoate
b. benzoic acid
Problem 21c,d
What products are obtained from the reaction of the following compounds with LiAlH4 followed by treatment with dilute acid?
c. methyl benzoate
d. pentanoic acid
Problem 22
What amides would you react with LiAlH4 to form the following amines?
a. benzylmethylamine
b. ethylamine
c. diethylamine
d. triethylamine
Problem 23a
How would you make the following compounds from N-benzylbenzamide?
a. dibenzylamine
Problem 23b
How would you make the following compounds from N-benzylbenzamide?
b. benzoic acid
Problem 23c
How would you make the following compounds from N-benzylbenzamide?
c. benzyl alcohol
Problem 24
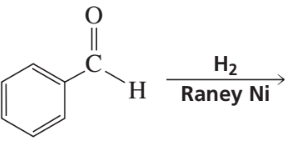
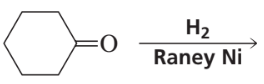
What are the products of the following reactions?
a.
b.
c.
d.
Problem 27
A ketone can be prepared from the reaction of a nitrile with a Grignard reagent. Describe the intermediate formed in this reaction, and show how it can be converted to a ketone.
Problem 28
Why is the pKa value of protonated hydroxylamine (6.0) so much lower than the value of a protonated primary amine such as protonated methylamine (10.7)?
Problem 30a
The pKa of protonated acetone is about –7.5 and the pKa of protonated hydroxylamine is 6.0.
a. In a reaction with hydroxylamine at pH 4.5 (Figure 16.2), what fraction of acetone is present in its acidic, protonated form?
<IMAGE>
Problem 31
Imines can exist as stereoisomers. The isomers are named using the E,Z system of nomenclature (Section 4.2 ). The lone pair has the lowest priority.
<IMAGE>
Draw the structure of each of the following compounds:
a. the (E)-hydrazone of benzaldehyde
b. the (Z)-oxime of propiophenone
Problem 33a,b
What are the products of the following reactions? (A trace amount of acid is present in each case.)
a. cyclopentanone + ethylamine
b. cyclopentanone + diethylamine