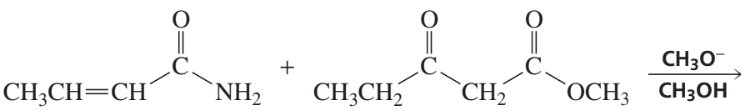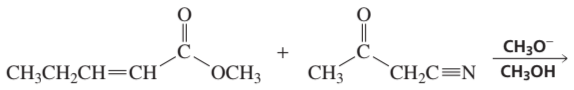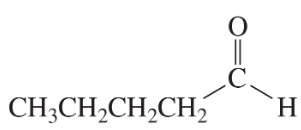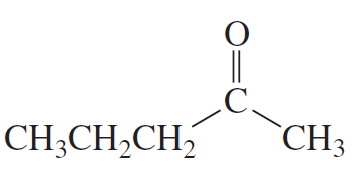Back
BackProblem 2a
Give an example for each of the following:
a. a β-keto nitrile
Problem 2b
Give an example for each of the following:
b. a β-diester
Problem 2c
Give an example for each of the following:
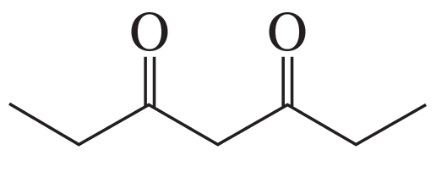
c. a β-keto aldehyde
Problem 3
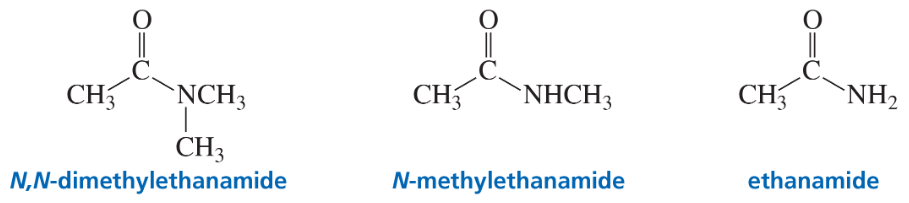
Explain why a base can remove a proton from the α-carbon of N,N-dimethylethanamide but not from the α-carbon of either N-methylethanamide or ethanamide.
Problem 4
Explain why the -hydrogen of an N,N-disubstituted amide is less acidic (pKa = 30) than the -hydrogen of an ester (pKa = 25).
Problem 5a
Rank the compounds in each of the following groups from strongest acid to weakest acid:
a.
Problem 5c
Rank the compounds in each of the following groups from strongest acid to weakest acid:
c.
Problem 6
Explain why 92% of 2,4-pentanedione exists as the enol tautomer in hexane but only 15% of this compound exists as the enol tautomer in water.
Problem 7d,e
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautomer, indicate the one that is more stable.
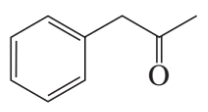
d.
e.
Problem 7f
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautomer, indicate the one that is more stable.
f.
Problem 10
A ketone undergoes acid-catalyzed bromination, acid-catalyzed chlorination, racemization, and acid-catalyzed deuterium exchange at the ⍺-carbon. All of these reactions have similar rate constants.What does this tell you about the mechanisms of these reactions?
Problem 11a
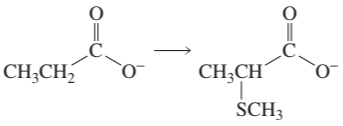
Show how the following compounds can be prepared from the given starting material:
a.
Problem 14a
How could each of the following compounds be prepared from a ketone and an alkyl halide?
a.
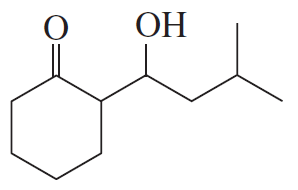
Problem 14b
How could each of the following compounds be prepared from a ketone and an alkyl halide?
b.
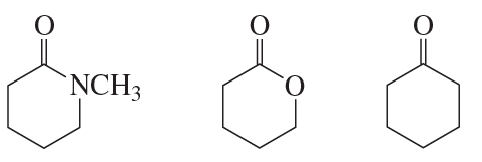
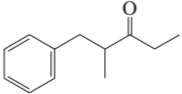
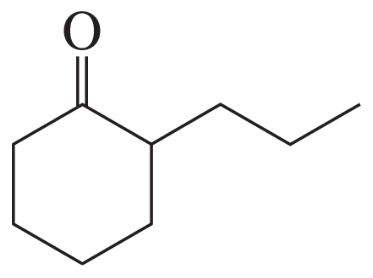
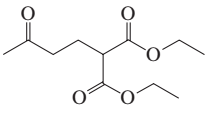
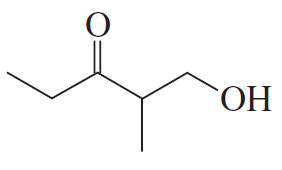
Problem 16a
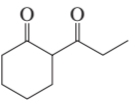
How could each of the following compounds be prepared from cyclohexanone?
a.
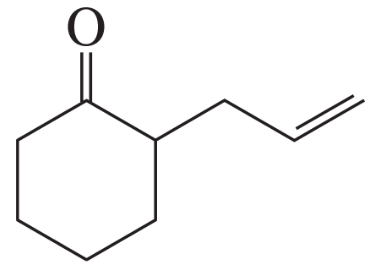
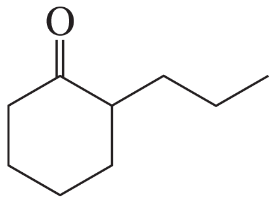
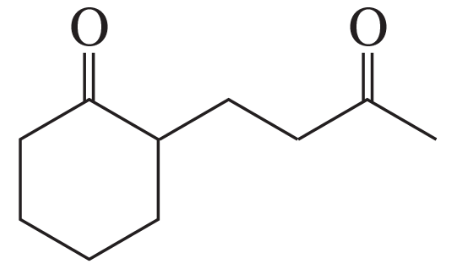
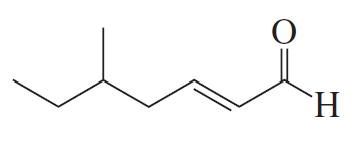
Problem 16b
How could each of the following compounds be prepared from cyclohexanone?
b.
Problem 17a
Describe how the following compounds could be prepared from cyclohexanone using an enamine intermediate:
a.
Problem 17b
Describe how the following compounds could be prepared from cyclohexanone using an enamine intermediate:
b.
Problem 18a
Draw the products of the following reactions:
a.
Problem 18b
Draw the products of the following reactions:
b.
Problem 19a
What reagents should be used to prepare the following compounds?
a.
Problem 19b
What reagents should be used to prepare the following compounds?
b.
Problem 19c
What reagents should be used to prepare the following compounds?
c.
Problem 20a
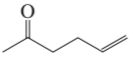
What aldol addition product is formed from each of the following compounds?
a.
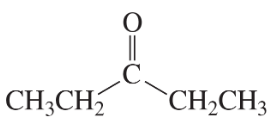
Problem 20b
What aldol addition product is formed from each of the following compounds?
b.
Problem 24
How could you prepare the following compound using a starting material that contains no more than three carbons?
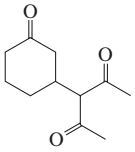
Problem 25a
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
a.
Problem 25b
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
b.
Problem 25c
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
c.
Problem 26
What two carbonyl compounds are required for the synthesis of morachalcone A, via a Claisen–Schmidt condensation?
<IMAGE>