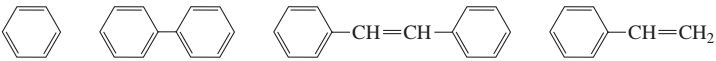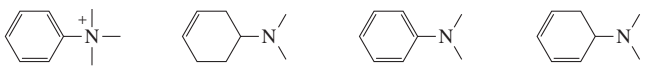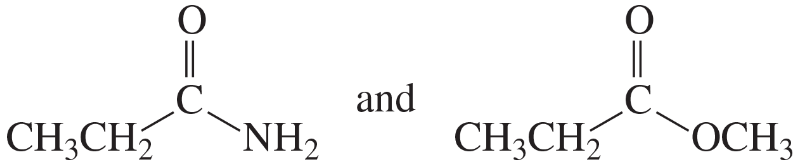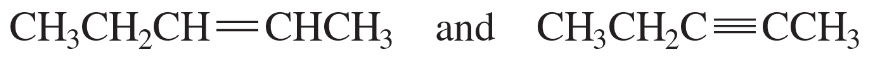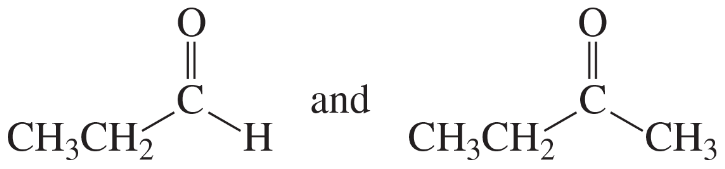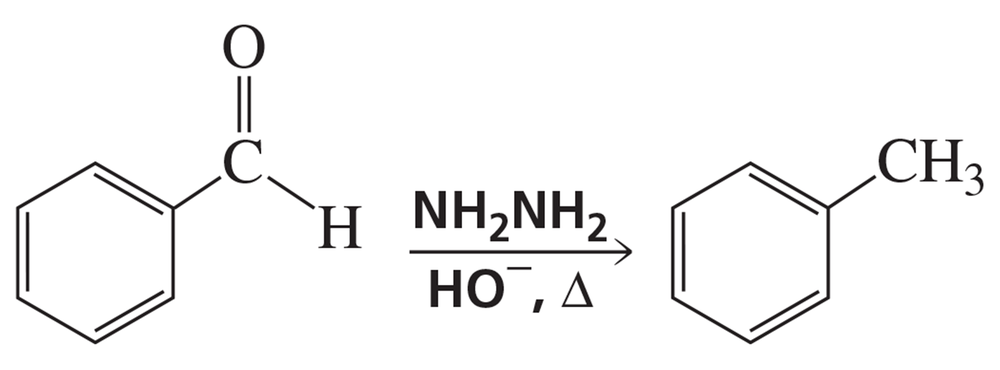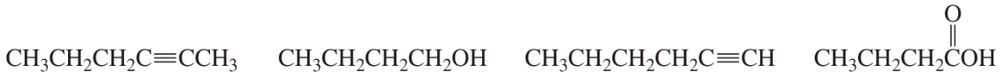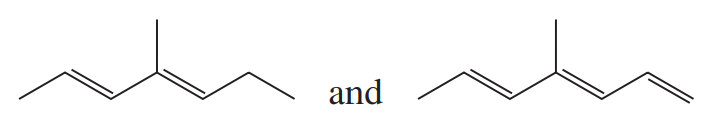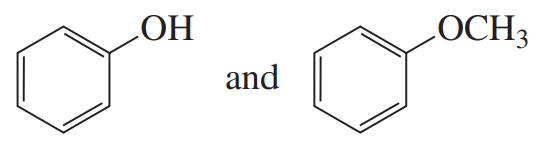Back
Back Bruice 8th Edition
Bruice 8th Edition Ch. 13 - Mass Spectrometry; Infrared Spectroscopy; UV/Vis Spectroscopy
Ch. 13 - Mass Spectrometry; Infrared Spectroscopy; UV/Vis SpectroscopyProblem 37a
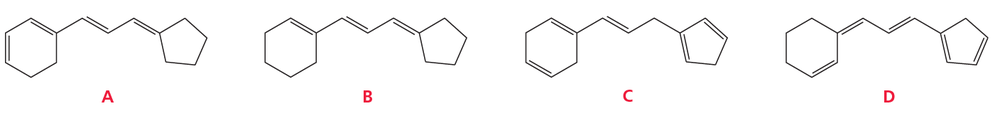
Rank each set of compounds in order of decreasing λmax:
a.
Problem 37b
Rank each set of compounds in order of decreasing λmax:
b.
Problem 38a
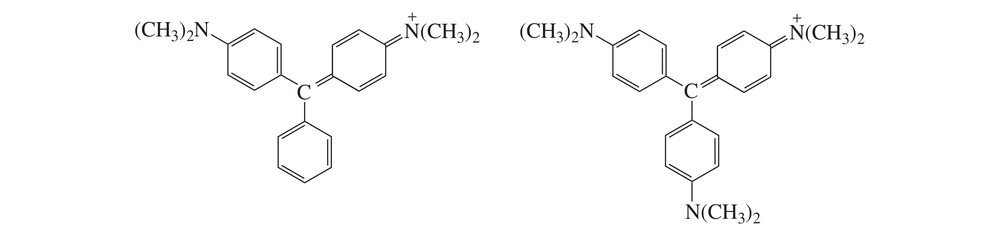
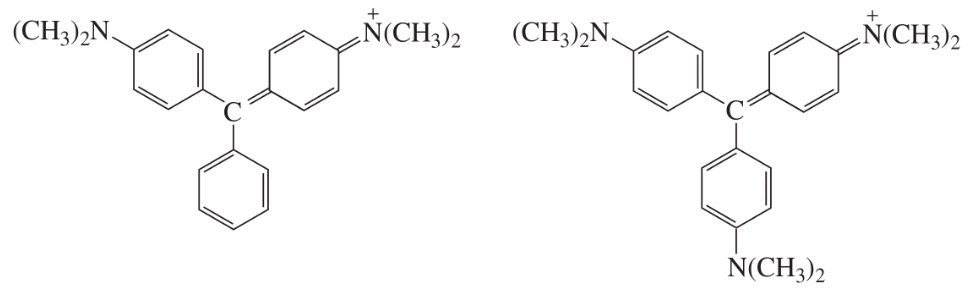
At pH = 7, one of the ions shown here is purple and the other is blue. Which is which?
Problem 38b
What would be the difference in the colors of the compounds at pH = 3?
Problem 41
In the mass spectrum of the following compounds, which is the tallest—the peak at m/z = 57 or the peak at m/z = 71?
a. 3-methylpentane
b. 2-methylpentane
Problem 44
Rank the following compounds in order of increasing lmax:
Problem 45c,d
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them:
c. CH3CH2CH2OH and CH3CH2OCH3
d.
Problem 45i,j
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them:
i.
j.
Problem 46
a. How could you use IR spectroscopy to determine whether the following reaction had occurred?
b. After purifying the product, how could you determine whether all the NH2NH2 had been removed?
Problem 47
Assuming that the force constant is approximately the same for C–C, C–N, and C–O bonds, predict the relative positions of their stretching vibrations in an IR spectrum.
Problem 48
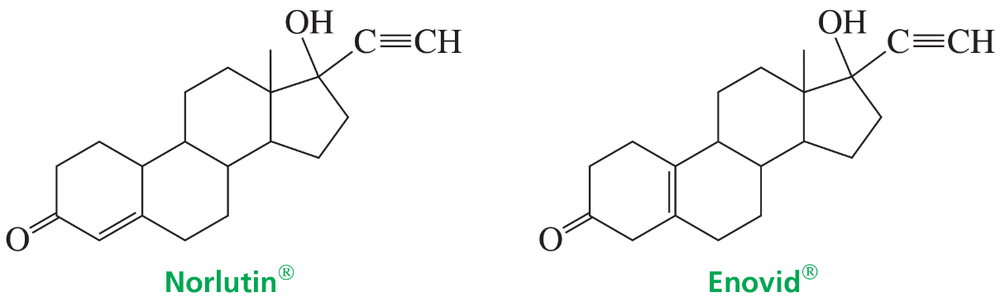
Norlutin and Enovid are ketones that suppress ovulation, so they have been used clinically as contraceptives. For which of these compounds would you expect the infrared carbonyl absorption (C=O stretch) to be at a higher frequency? Explain.
Problem 50
A mass spectrum shows significant peaks at m/z = 87, 115, 140, and 143. Which of the following compounds is responsible for that mass spectrum?
Problem 52
A compound gives a mass spectrum with essentially only three peaks at m/z = 77 (40%), 112 (100%), and 114 (33%). Identify the compound.
Problem 53
What hydrocarbons that contain a six-membered ring will have a molecular ion peak at m/z = 112?
Problem 55
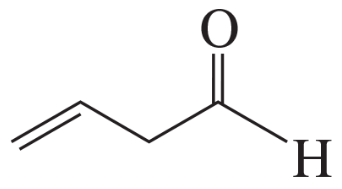
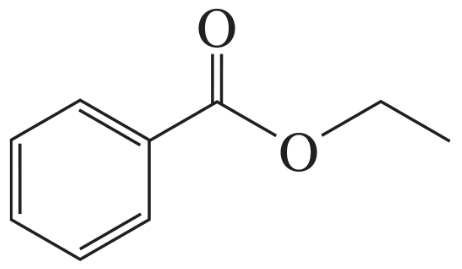
Rank the following compounds from highest wavenumber to lowest wavenumber for their C=O absorption band:
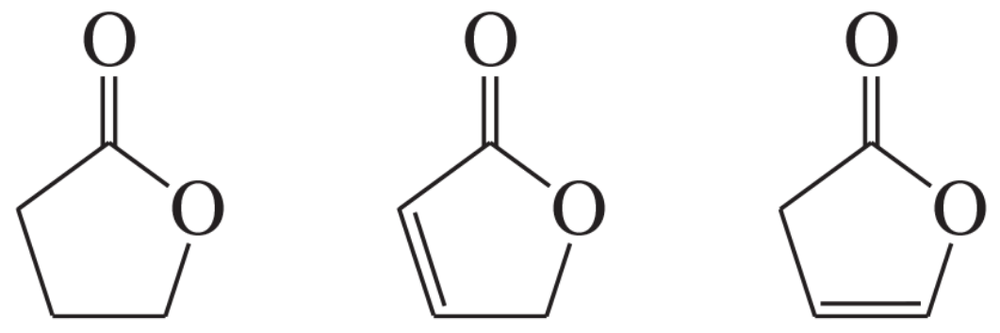
Problem 56
Rank the following compounds from highest wavenumber to lowest wavenumber for their C-O absorption band:
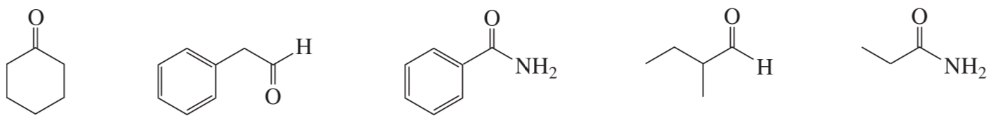
Problem 58a
The mass spectrum for a compound with molecular weight of 102 is shown below. Its IR spectrum has a broad, strong absorption at 3600 cm–1 and a medium absorption at 1360 cm–1.
a. Identify the compound.
<IMAGE>
Problem 58b
The mass spectrum for a compound with molecular weight of 102 is shown below. Its IR spectrum has a broad, strong absorption at 3600 cm–1 and a medium absorption at 1360 cm–1.
b. Show the mechanism for formation of the peak at m/z = 84.
<IMAGE>
Problem 59
Which one of the following five compounds produced the IR spectrum shown below?
<IMAGE>
Problem 60
The IR spectrum of compound A with a molecular formula of C5H12O is shown below. Compound A is oxidized to give compound B, a ketone with a molecular formula of C5H10O. When compound A is heated with H2SO4, compounds C and D are obtained. Considerably more D is obtained than C. Reaction of compound C with O3, followed by treatment with dimethyl sulfide, gives two products: formaldehyde and compound E, with a molecular formula of C4H8O. Reaction of compound D with O3, followed by treatment with dimethyl sulfide, gives two products: compound F, with a molecular formula of C3H6O, and compound G, with a molecular formula of C2H4O. What are the structures of compounds A through G?
<IMAGE>
Problem 61a
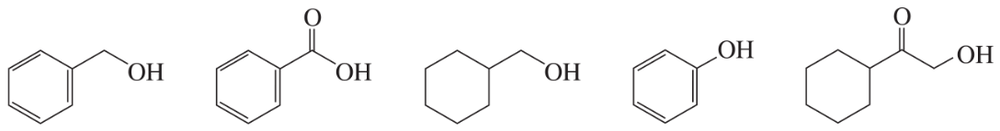
Each of the IR spectra shown below is accompanied by a set of four compounds. In each case, indicate which of the four compounds is responsible for the spectrum.
a.
<IMAGE>
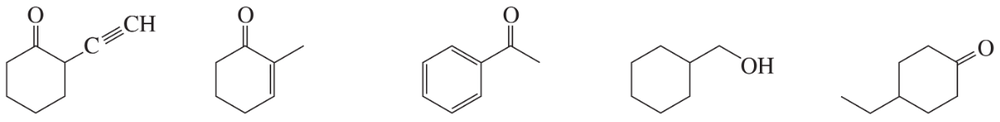
Problem 62c
Five compounds are shown for each of the IR spectra below. Indicate which of the five compounds is responsible for each spectrum.
c.
<IMAGE>
Problem 64
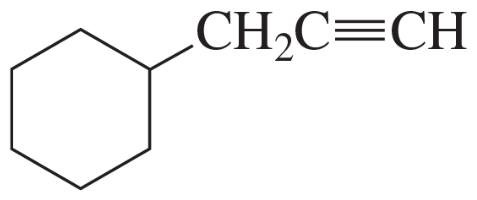
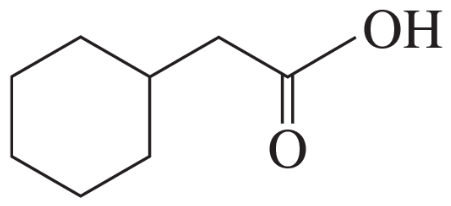
How can IR spectroscopy distinguish between 1-hexyne, 2-hexyne, and 3-hexyne?
Problem 67a,b
Give approximate wavenumbers for the major characteristic IR absorption bands that would be given by each of the following compounds:
a.
b.
Problem 67e,f
Give approximate wavenumbers for the major characteristic IR absorption bands that would be given by each of the following compounds:
e.
f.
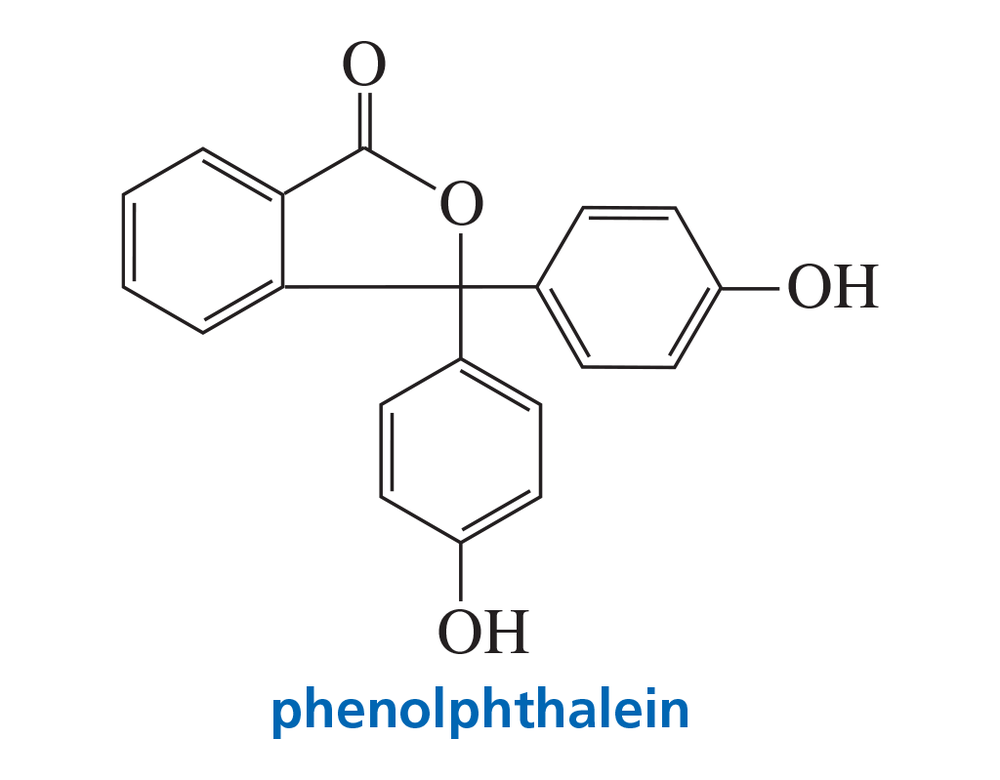
Problem 70
Phenolphthalein is an acid–base indicator. In solutions of pH < 8.5, it is colorless; in solutions of pH > 8.5, it is deep red-purple. Account for the change in color.
Problem 71a
Each of the IR spectra shown below is the spectrum of one of the following compounds. Identify the compound that produced each spectrum.
a. <IMAGE>
Problem 71b
Each of the IR spectra shown below is the spectrum of one of the following compounds. Identify the compound that produced each spectrum.
b. <IMAGE>
Problem 72a
How can you use UV spectroscopy to distinguish between the compounds in each of the following pairs?
a.
Problem 72b
How can you use UV spectroscopy to distinguish between the compounds in each of the following pairs?
b.