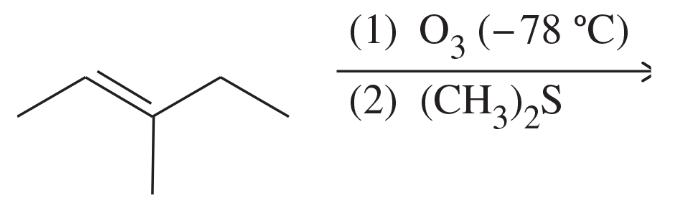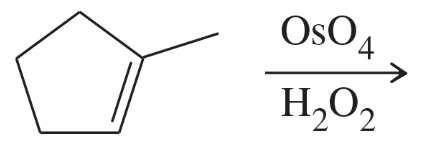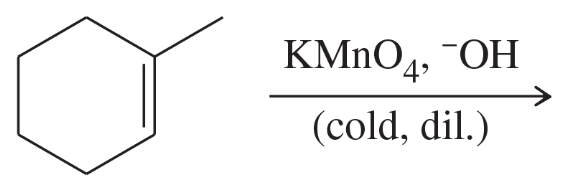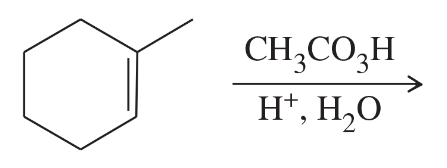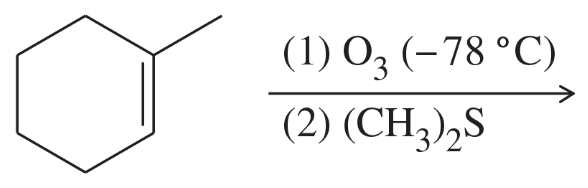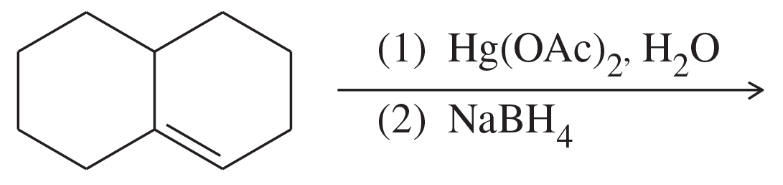Back
BackProblem 46d
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
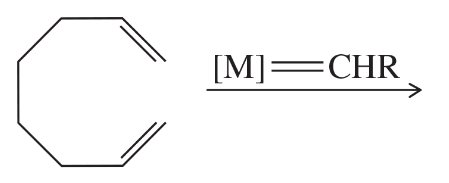
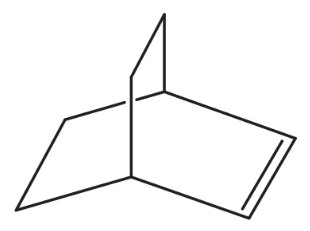
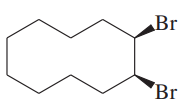
(d)
Problem 46h
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(h)
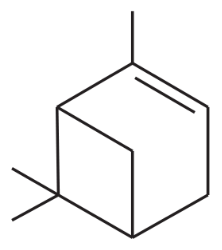
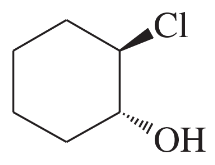
Problem 46i
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(i)
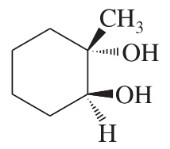
Problem 46j
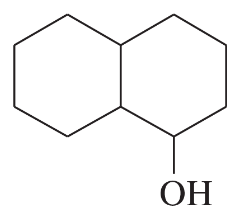
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
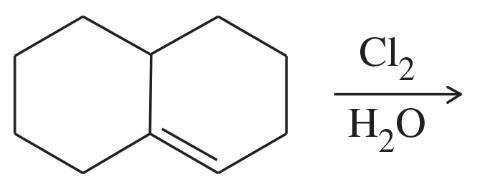
(j)
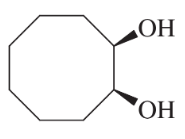
Problem 46k
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
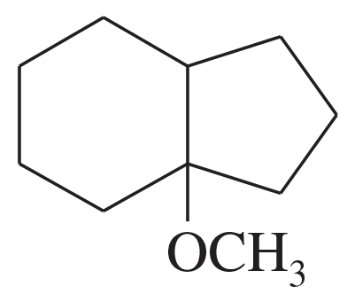
(k)
Problem 46l
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(l)
Problem 46m
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(m)
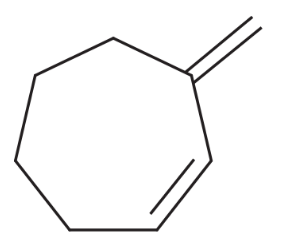
Problem 46n
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate. n
(n)
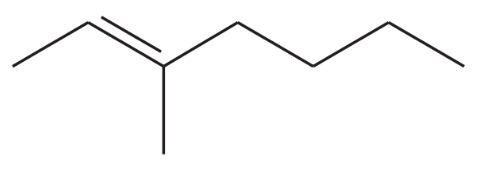
Problem 46o
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(o)
Problem 46p
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(p)
Problem 46q
Predict the major products of the following reactions, and give the structures of any intermediates. Include stereochemistry where appropriate.
(q)
Problem 47a
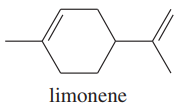
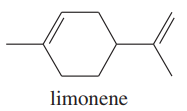
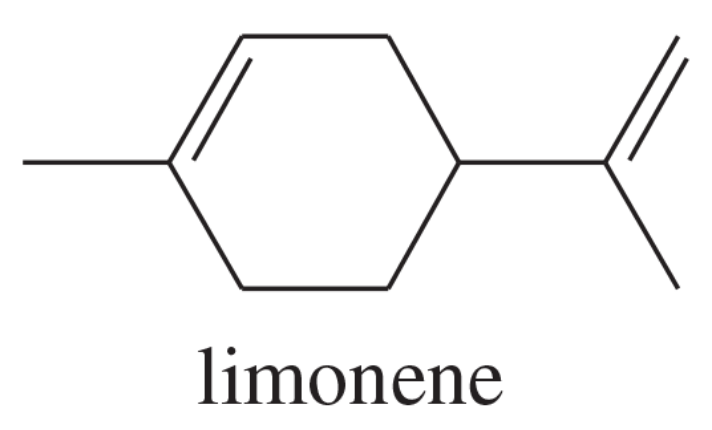
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
a. borane in tetrahydrofuran, followed by basic hydrogen peroxide
Problem 47b
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
b. m-chloroperoxybenzoic acid
Problem 47c
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
(c) ozone, then dimethyl sulfide
Problem 47d
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
d. a mixture of osmic acid and hydrogen peroxide
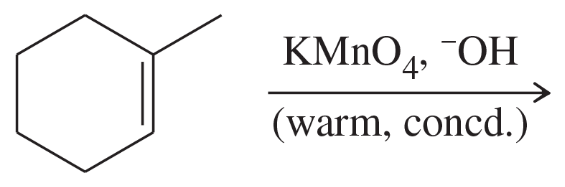
Problem 47e
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
(e) hot, concentrated potassium permanganate
Problem 47f
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
f. peroxyacetic acid in acidic water
Problem 47i
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
(i) hydrogen bromide gas in a solution containing dimethyl peroxide
Problem 47m
Limonene is one of the compounds that give lemons their tangy odor. Show the structures of the products expected when limonene reacts with an excess of each of these reagents.
m. CHBr3 and 50% aq. NaOH
Problem 48a,b
Give the products expected when the following compounds are ozonized and reduced.
(a)
(b)
Problem 48c,d
Give the products expected when the following compounds are ozonized and reduced.
(c)
(d)
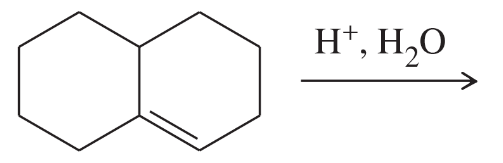
Problem 49a
Show how you would make the following compounds from a suitable cyclic alkene.
(a)
Problem 49b
Show how you would make the following compounds from a suitable cyclic alkene.
(b)
Problem 49c
Show how you would make the following compounds from a suitable cyclic alkene.
(c)
Problem 49d
Show how you would make the following compounds from a suitable cyclic alkene.
(d)
Problem 49e
Show how you would make the following compounds from a suitable cyclic alkene.
(e)
Problem 49f
Show how you would make the following compounds from a suitable cyclic alkene.
(f)
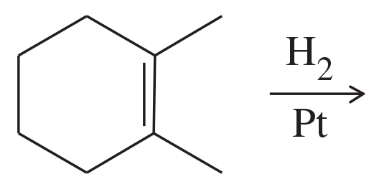
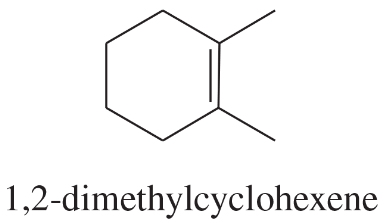
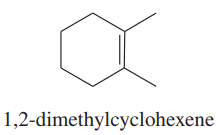
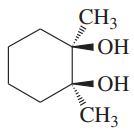
Problem 50a
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(a)
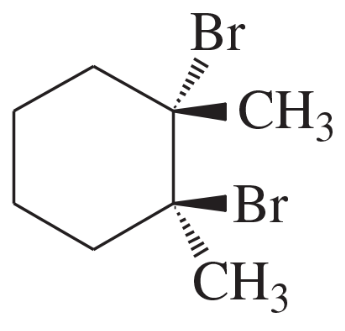
Problem 50c
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(c)
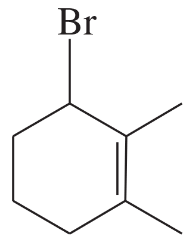
Problem 50d
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(d)