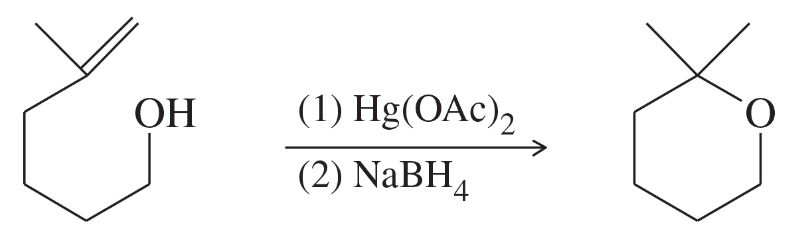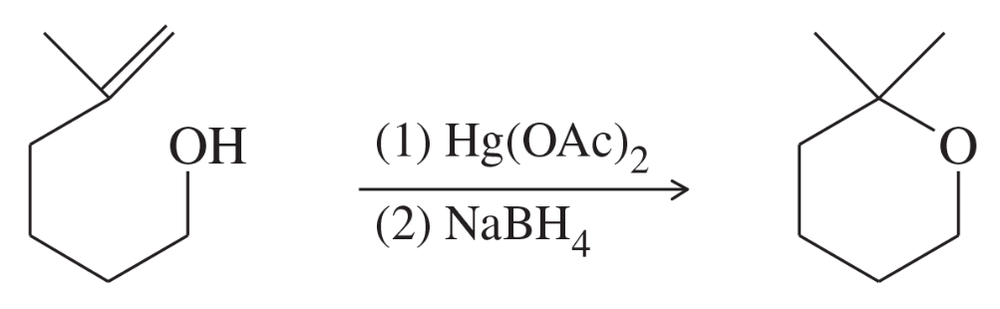Back
BackProblem 60
Unknown X, C5H9Br does not react with bromine or with dilute KMnO4. Upon treatment with potassium tert-butoxide, X gives only one product, Y, C5H8. Unlike X, Y decolorizes bromine and changes KMnO4 from purple to brown. Catalytic hydrogenation of Y gives methylcyclobutane. Ozonolysis–reduction of Y gives dialdehyde Z, C5H8O2. Propose consistent structures for X, Y, and Z. Is there any aspect of the structure of X that is still unknown?
Problem 61
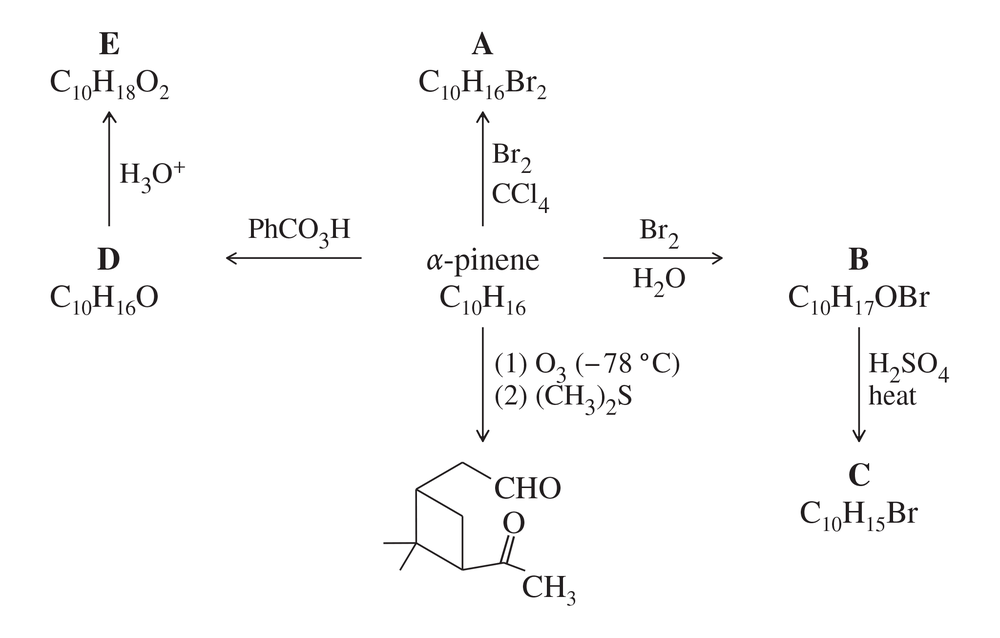
One of the constituents of turpentine is α-pinene, formula C10H6. The following scheme (called a “road map”) gives some reactions of α-pinene. Determine the structure of α-pinene and of the reaction products of A through E.
Problem 62
The sex attractant of the housefly has the formula C23H46. When treated with warm potassium permanganate, this pheromone gives two products: CH3(CH2)12COOH and CH3(CH2)7COOH. Suggest a structure for this sex attractant. Explain which part of the structure is uncertain.
Problem 63
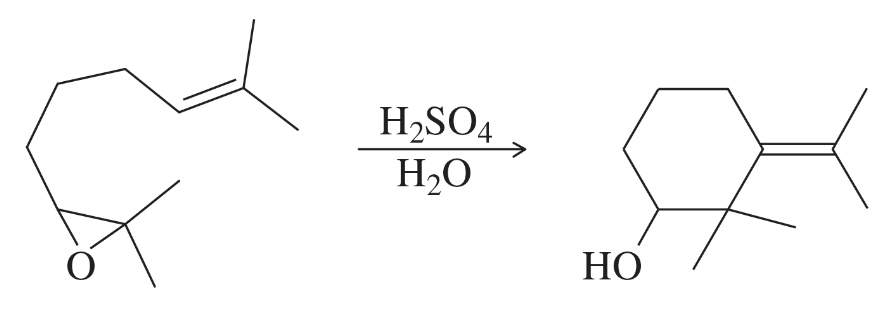
In contact with a platinum catalyst, an unknown alkene reacts with three equivalents of hydrogen gas to give 1-isopropyl-4-methylcyclohexane. When the unknown alkene is ozonized and reduced, the products are the following:

Deduce the structure of the unknown alkene.
Problem 64
Propose a mechanism for the following reaction.
Problem 65a,b
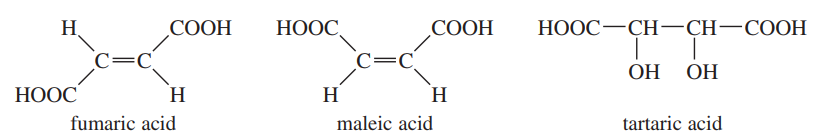
The two butenedioic acids are called fumaric acid (trans) and maleic acid (cis). 2,3-Dihydroxybutanedioic acid is called tartaric acid.
Show how you would convert
a. fumaric acid to (±)-tartaric acid.
b. fumaric acid to meso-tartaric acid.
Problem 65b
The two butenedioic acids are called fumaric acid (trans) and maleic acid (cis). 2,3-Dihydroxybutanedioic acid is called tartaric acid.
Show how you would convert
b. fumaric acid to meso-tartaric acid.
Problem 65c
The two butenedioic acids are called fumaric acid (trans) and maleic acid (cis). 2,3-Dihydroxybutanedioic acid is called tartaric acid.
Show how you would convert
c. maleic acid to (±)-tartaric acid.
Problem 68
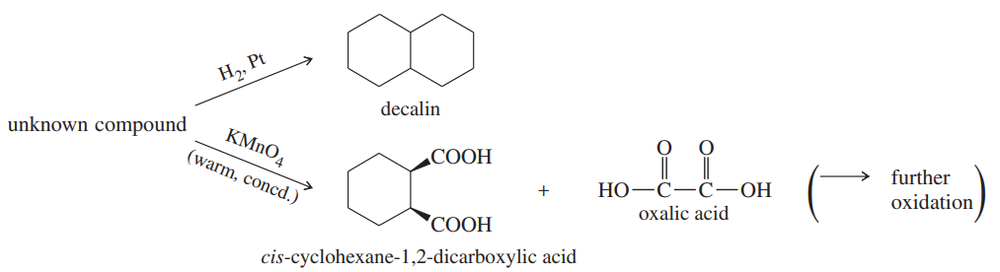
An unknown compound decolorizes bromine in carbon tetrachloride, and it undergoes catalytic reduction to give decalin. When treated with warm, concentrated potassium permanganate, this compound gives cis-cyclohexane-1,2-dicarboxylic acid and oxalic acid. Propose a structure for the unknown compound.
Problem 70a
The following cyclization has been observed in the oxymercuration-demercuration of this unsaturated alcohol. Propose a mechanism for this reaction.
Problem 70b
Predict the product of formula C7H13BrO from the reaction of this same unsaturated alcohol with bromine. Propose a mechanism to support your prediction.
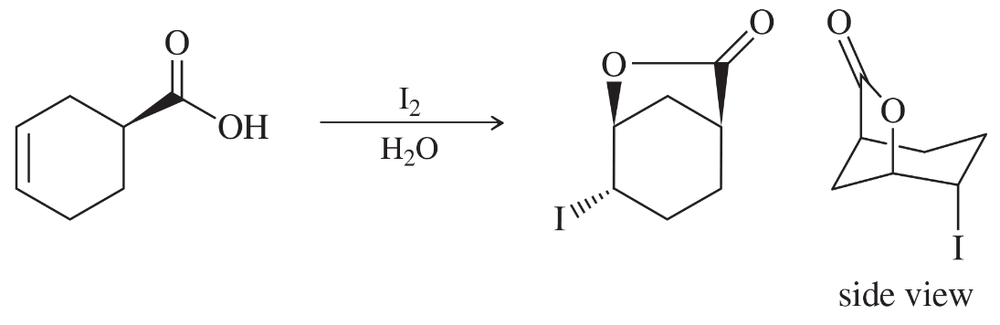
Problem 71
A graduate student attempted to form the iodohydrin of the alkene shown below. Her analysis of the products showed a good yield of an unexpected product. Propose a mechanism to explain the formation of this product.
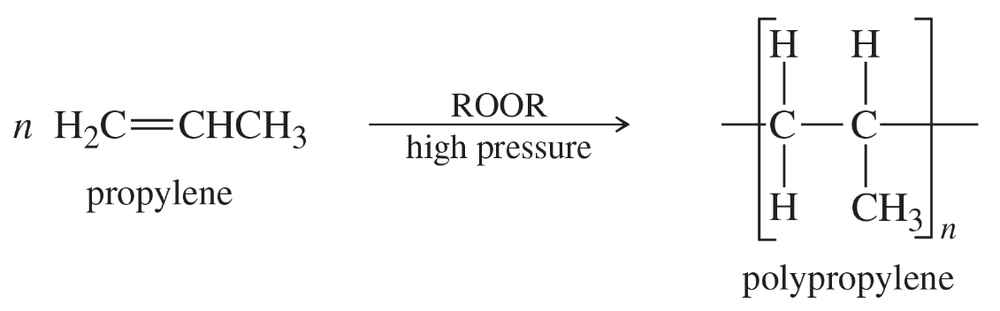
Problem 72
Propose a mechanism for reaction of the first three propylene units in the polymerization of propylene in the presence of a peroxide.
Problem 73
When styrene (vinylbenzene) is commercially polymerized, about 1–3% of 1,4-divinylbenzene is often added to the styrene. The incorporation of some divinylbenzene gives a polymer with more strength and better resistance to organic solvents. Explain how a very small amount of divinylbenzene has a marked effect on the properties of the polymer.
Problem 74
The cationic polymerization of isobutylene (2-methylpropene) is shown in Section 8-16A. Isobutylene is often polymerized under free-radical conditions. Propose a mechanism for the free-radical polymerization of isobutylene.
Problem 75
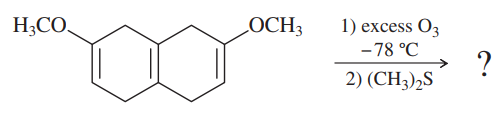
Ozonolysis can be applied selectively to different types of carbon–carbon double bonds. The compound shown below contains two vinyl ether double bonds, which are electron-rich because of the electron-donating alkoxy groups. Ozone reacts more quickly with electron-rich double bonds and more slowly with hindered double bonds. At −78 °C, this compound quickly adds two equivalents of ozone. Immediate reduction of the ozonide gives a good yield of a single product. Show the expected ozonolyis product, and label the functional groups produced, some of which are not typical from ozonolysis of simple alkenes.
Problem 76
Propose mechanisms to explain the opposite regiochemistry observed in the following two reactions.
Problem 77
An inexperienced graduate student treated dec-5-ene with borane in THF, placed the flask in a refrigerator, and left for a party. When he returned from the party, he discovered that the refrigerator was broken and that it had gotten quite warm inside. Although all the THF had evaporated from the flask, he treated the residue with basic hydrogen peroxide. To his surprise, he recovered a fair yield of decan-1-ol. Use a mechanism to show how this reaction might have occurred. (Hint: The addition of BH3 is reversible.)
Problem 78
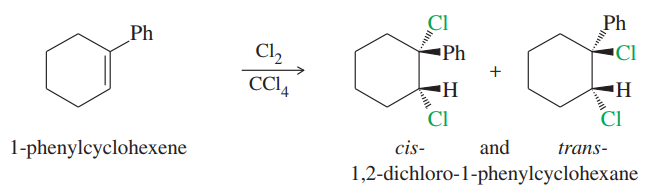
We have seen many examples where halogens add to alkenes with anti stereochemistry via the halonium ion mechanism. However, when 1-phenylcyclohexene reacts with chlorine in carbon tetrachloride, a mixture of the cis and trans isomers of the product is recovered. Propose a mechanism, and explain this lack of stereospecificity.
Problem 79a
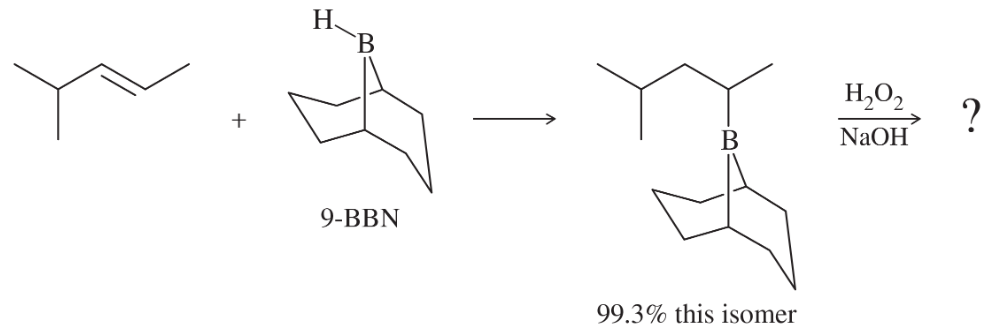
The bulky borane 9-BBN was developed to enhance the selectivity of hydroboration. In this example, 9-BBN adds to the less hindered carbon with 99.3% regioselectivity, compared with only 57% for diborane.
a. Show the two organic products generated when the trialkylborane is oxidized with H2O2/NaOH.
Problem 79b
The bulky borane 9-BBN was developed to enhance the selectivity of hydroboration. In this example, 9-BBN adds to the less hindered carbon with 99.3% regioselectivity, compared with only 57% for diborane.
b. 9-BBN is synthesized by adding BH3 across a symmetric, cyclic diene. What is the structure of the diene?