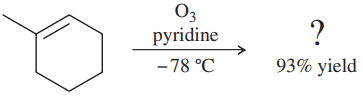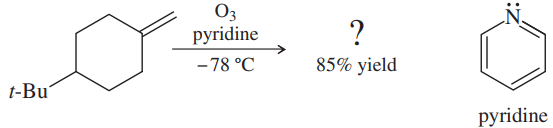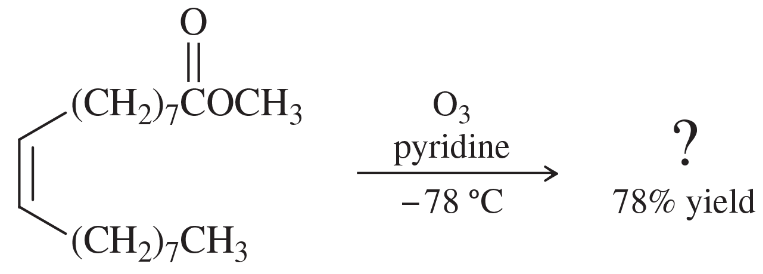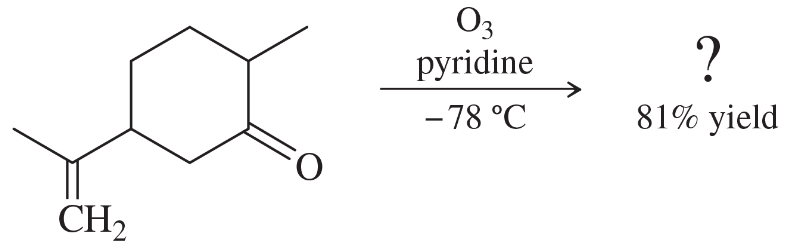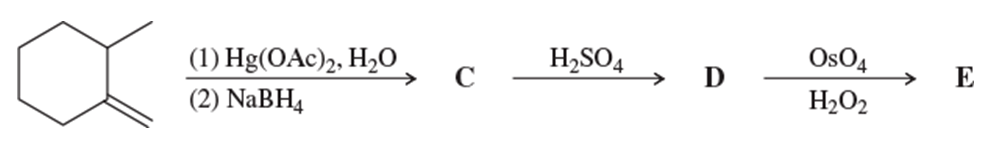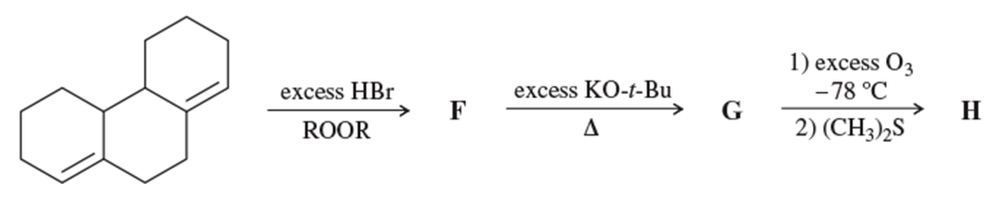Back
BackProblem 50e
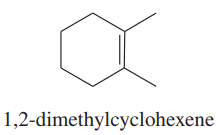
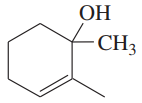
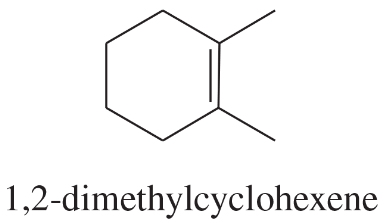
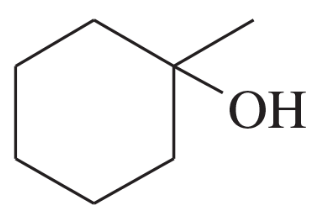
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
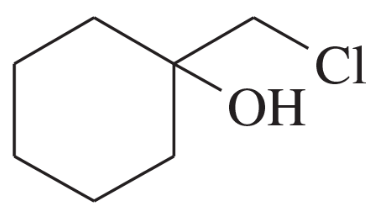
(e)
Problem 50g
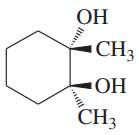
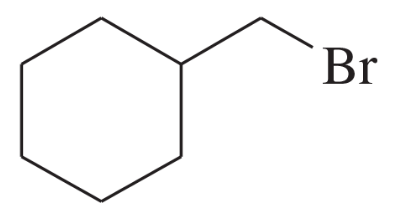
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(g)
Problem 50h
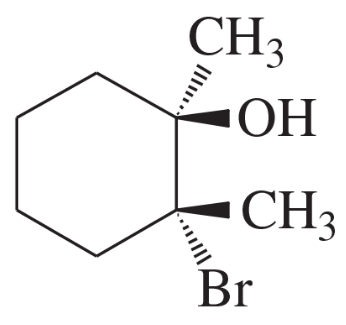
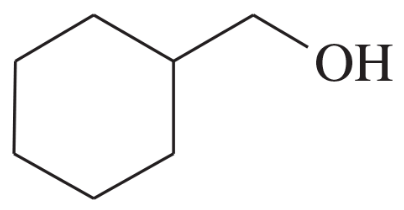
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
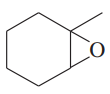
(h)
Problem 50i
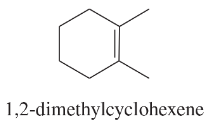
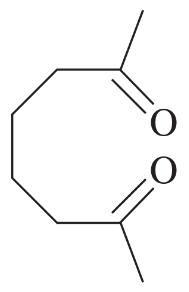
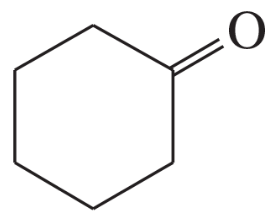
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(i)
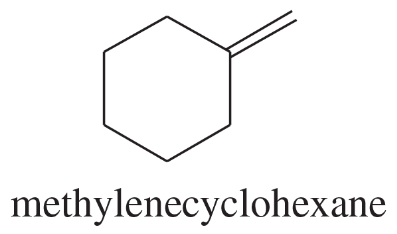
Problem 51a
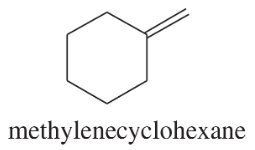
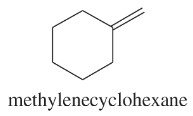
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(a)
Problem 51b
Show how you would synthesize each compound using methylenecyclopentane as your starting material.
(b)
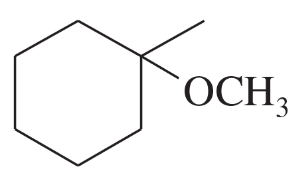
Problem 51c
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(c)
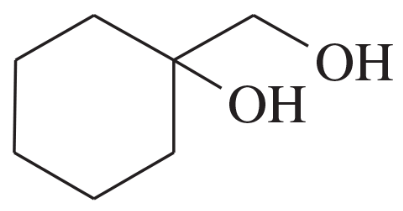
Problem 51d
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(d)
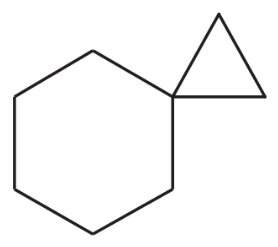
Problem 51e
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(e)
Problem 51f
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(f)
Problem 51g
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(g)
Problem 51h
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(h)
Problem 51i
Show how you would synthesize each compound using methylenecyclohexane as your starting material.
(i)
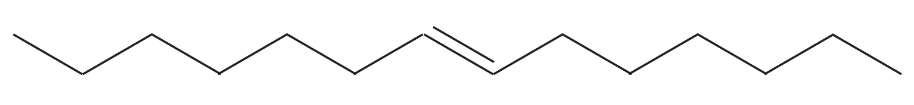
Problem 52a
Show what products you would expect from the following metathesis reactions, using the Schrock or Grubbs catalysts.
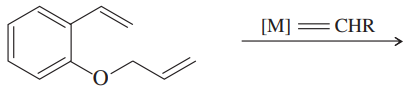
(a)
Problem 52b
Show what products you would expect from the following metathesis reactions, using the Schrock or Grubbs catalysts.
(b)
Problem 52c
Show what products you would expect from the following metathesis reactions, using the Schrock or Grubbs catalysts.
(c)
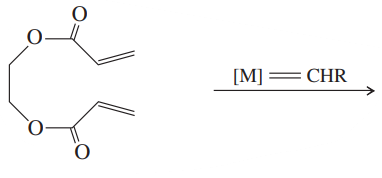
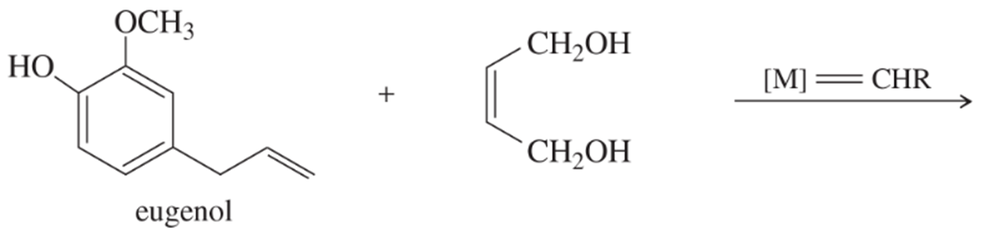
Problem 53
Show how you might use olefin metathesis to assemble the following alkenes from smaller units:
(a)
(b)
Problem 54a,b
Professor Patrick Dussault (University of Nebraska at Lincoln) has developed an alternative to the standard two-step ozonolysis procedure requiring reduction of the ozonide in a second step. He uses 2 to 3 equivalents of pyridine, a mildly basic organic solvent, in a one-step process (Organic Letters, 2012, 14, 2242). Show the products you expect from the following examples.
(a)
(b)
Problem 54c,d
Professor Patrick Dussault (University of Nebraska at Lincoln) has developed an alternative to the standard two-step ozonolysis procedure requiring reduction of the ozonide in a second step. He uses 2 to 3 equivalents of pyridine, a mildly basic organic solvent, in a one-step process (Organic Letters, 2012, 14, 2242). Show the products you expect from the following examples.
(c)
(d)
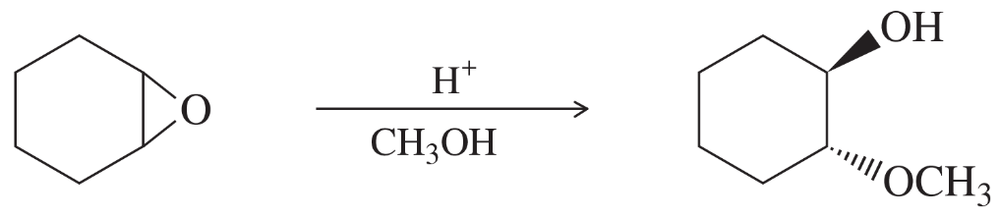
Problem 55a
Complete each synthesis by providing the structure of the major product at each step, including any important stereochemistry.
a.
Problem 55b
Following the instructions for drawing the energy levels of the molecular orbitals for the compounds shown in [Figure 8.17], draw the energy levels of the molecular orbitals for the cycloheptatrienyl cation. For each compound, show the distribution of the electrons. Which of the compounds are aromatic?
Problem 55b,c,d
Complete each synthesis by providing the structure of the major product at each step, including any important stereochemistry.
b.
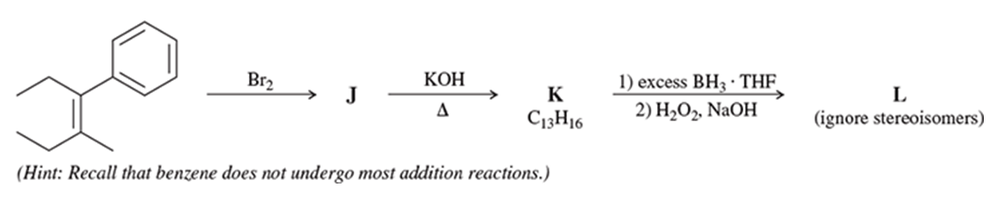
c.
d.
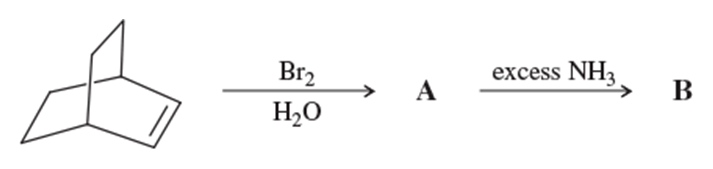
Problem 56a
Propose mechanisms consistent with the following reactions.
(a)
Problem 56b
Propose mechanisms consistent with the following reactions.
(b)
Problem 56c
Propose mechanisms consistent with the following reactions.
(c)
Problem 56d
Propose mechanisms consistent with the following reactions.
(d)
Problem 56f
Propose mechanisms consistent with the following reactions.
(f)
Problem 56g
Propose mechanisms consistent with the following reactions.
(g)
Problem 57
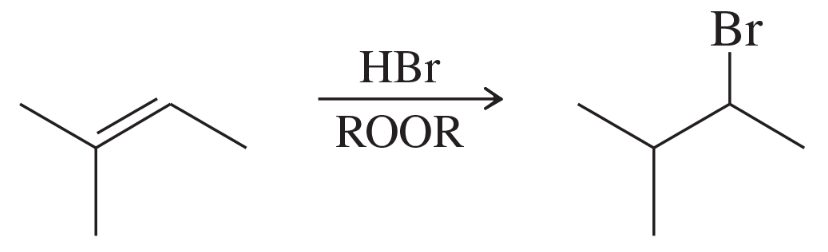
Draw an approximate reaction-energy diagram showing the curves for the two possible pathways for ionic addition of HBr to 1-methylcyclohexene. (a) Formation of the major product, 1-bromo-1-methylcyclohexane, and (b) formation of the minor product, 1-bromo-2-methylcyclohexane. Point out how these curves show that 1-bromo-1-methylcyclohexane should be formed faster.
Problem 58
Cyclohexene is dissolved in a solution of lithium chloride in chloroform. To this solution is added one equivalent of bromine. The material isolated from this reaction contains primarily a mixture of trans-1,2-dibromocyclohexane and trans-1-bromo-2-chlorocyclohexane. Propose a mechanism to show how these compounds are formed.