Back
BackProblem 25c
What do the results of parts (a) and (b) imply about the hemiacetal structure of fructose?
Problem 26
Propose a mechanism for methylation of any one of the hydroxy groups of methyl α-D-glucopyranoside, using NaOH and dimethyl sulfate.
Problem 27a
Draw the expected product of the reaction of the following sugars with excess methyl iodide and silver oxide.
(a) α-D-fructofuranose
Problem 27b
Draw the expected product of the reaction of the following sugars with excess methyl iodide and silver oxide.
(b) β-D-galactopyranose
Problem 28a
Predict the products formed when the following sugars react with excess acetic anhydride and pyridine.
(a) α-D-glucopyranose
Problem 28b
Predict the products formed when the following sugars react with excess acetic anhydride and pyridine.
(b) β-D-ribofuranose
Problem 29
Show that Ruff degradation of D-mannose gives the same aldopentose (D-arabinose) as does D-glucose.
Problem 30
D-Lyxose is formed by Ruff degradation of galactose. Give the structure of D-lyxose. Ruff degradation of D-lyxose gives D-threose. Give the structure of D-threose.
Problem 31
D-Altrose is an aldohexose. Ruff degradation of D-altrose gives the same aldopentose as does degradation of D-allose, the C3 epimer of glucose. Give the structure of D-altrose.
Problem 32
Ruff degradation of D-arabinose gives D-erythrose. The Kiliani–Fischer synthesis converts D-erythrose to a mixture of D-arabinose and D-ribose. Draw out these reactions, and give the structure of D-ribose.
Problem 33
The Wohl degradation, an alternative to the Ruff degradation, is nearly the reverse of the Kiliani–Fischer synthesis. The aldose carbonyl group is converted to the oxime, which is dehydrated by acetic anhydride to the nitrile (a cyanohydrin). Cyanohydrin formation is reversible, and a basic hydrolysis allows the cyanohydrin to lose HCN. Using the following sequence of reagents, give equations for the individual reactions in the Wohl degradation of D-arabinose to D-erythrose. Mechanisms are not required.
a. hydroxylamine hydrochloride
b. acetic anhydride
c. –OH, H2O
Problem 35a
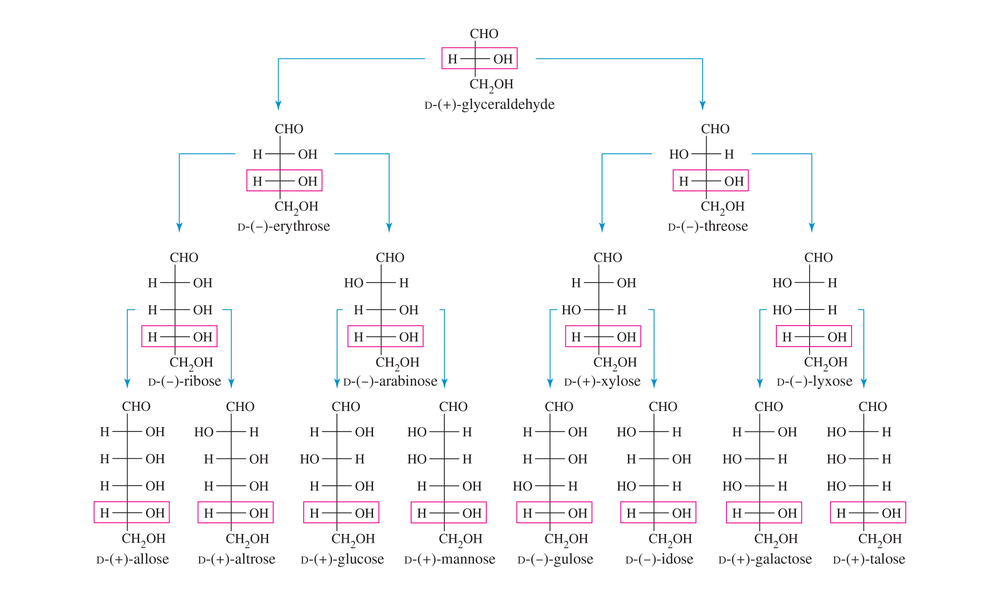
In 1891, Emil Fischer determined the structures of glucose and the seven other D-aldohexoses using only simple chemical reactions and clever reasoning about stereochemistry and symmetry. He received the Nobel Prize for this work in 1902. Fischer had determined that D-glucose is an aldohexose, and he used Ruff degradations to degrade it to (+)-glyceraldehyde. Therefore, the eight D-aldohexose structures shown in Figure 23-3 are the possible structures for glucose.
Pretend that no names are shown in Figure 23-3 except for glyceraldehyde, and use the following results to prove which of these structures represent glucose, mannose, arabinose, and erythrose.
(a) Upon Ruff degradation, glucose and mannose give the same aldopentose: arabinose. Nitric acid oxidation of arabinose gives an optically active aldaric acid. What are the two possible structures of arabinose?
Problem 35b
In 1891, Emil Fischer determined the structures of glucose and the seven other D-aldohexoses using only simple chemical reactions and clever reasoning about stereochemistry and symmetry. He received the Nobel Prize for this work in 1902. Fischer had determined that D-glucose is an aldohexose, and he used Ruff degradations to degrade it to (+)-glyceraldehyde. Therefore, the eight D-aldohexose structures shown in Figure 23-3 are the possible structures for glucose.
Pretend that no names are shown in Figure 23-3 except for glyceraldehyde, and use the following results to prove which of these structures represent glucose, mannose, arabinose, and erythrose.
(b) Upon Ruff degradation, arabinose gives the aldotetrose erythrose. Nitric acid oxidation of erythrose gives an optically inactive aldaric acid, meso-tartaric acid. What is the structure of erythrose?
Problem 36
Draw the structures of the individual mutarotating α and β anomers of maltose.
Problem 37
Give an equation to show the reduction of Tollens reagent by maltose.
Problem 38
Does lactose mutarotate? Is it a reducing sugar? Explain. Draw the two anomeric forms of lactose
Problem 39
Is gentiobiose a reducing sugar? Does it mutarotate? Explain your reasoning
Problem 40
Trehalose is a nonreducing disaccharide (C12H22O11) isolated from the poisonous mushroom Amanita muscaria. Treatment with an α-glucosidase converts trehalose to two molecules of glucose, but no reaction occurs when trehalose is treated with a β-glucosidase. When trehalose is methylated by dimethyl sulfate in mild base and then hydrolyzed, the only product is 2,3,4,6-tetra-O-methylglucose. Propose a complete structure and systematic name for trehalose.
Problem 41
Raffinose is a trisaccharide (C18H32O16) isolated from cottonseed meal. Raffinose does not reduce Tollens reagent, and it does not mutarotate. Complete hydrolysis of raffinose gives D-glucose, D-fructose, and D-galactose. When raffinose is treated with invertase, the products are D-fructose and a reducing disaccharide called melibiose. Raffinose is unaffected by treatment with a β-galactosidase, but an α-galactosidase hydrolyzes it to D-galactose and sucrose. When raffinose is treated with dimethyl sulfate and base followed by hydrolysis, the products are 2,3,4-tri-O-methylglucose, 1,3,4,6-tetra-O-methylfructose, and 2,3,4,6-tetra-O-methylgalactose. Determine the complete structures of raffinose and melibiose, and give a systematic name for melibiose.
Problem 42
Cellulose is converted to cellulose acetate by treatment with acetic anhydride and pyridine. Cellulose acetate is soluble in common organic solvents, and it is easily dissolved and spun into fibers. Show the structure of cellulose acetate.
Problem 43
Cytosine, uracil, and guanine have tautomeric forms with aromatic hydroxy groups. Draw these tautomeric forms.
Problem 44a
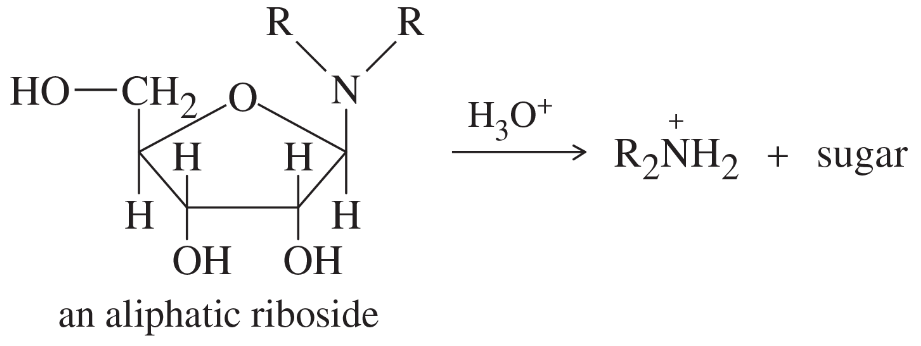
An aliphatic aminoglycoside is relatively stable to base, but it is quickly hydrolyzed by dilute acid. Propose a mechanism for the acid-catalyzed hydrolysis.
Problem 44b
Ribonucleosides are not so easily hydrolyzed, requiring relatively strong acid. Using your mechanism for part (a), show why cytidine and adenosine (for example) are not so readily hydrolyzed. Explain why this stability is important for living organisms.
Problem 45
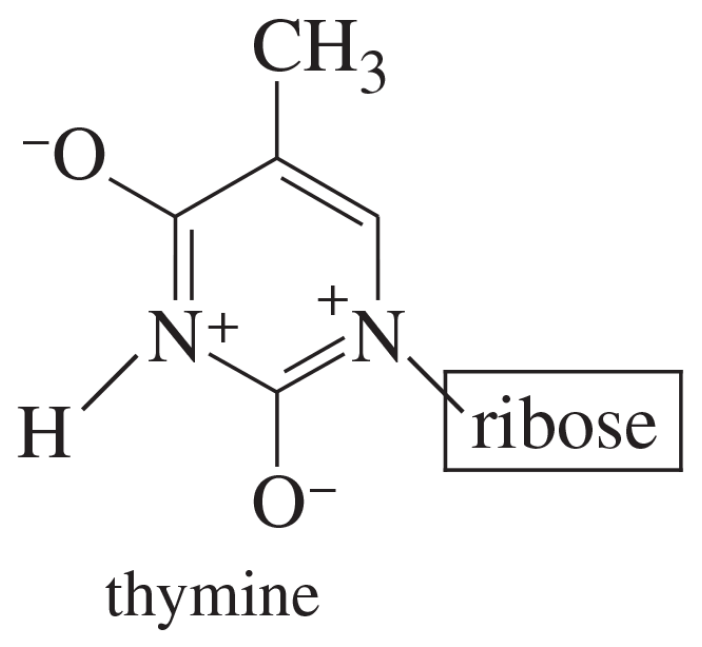
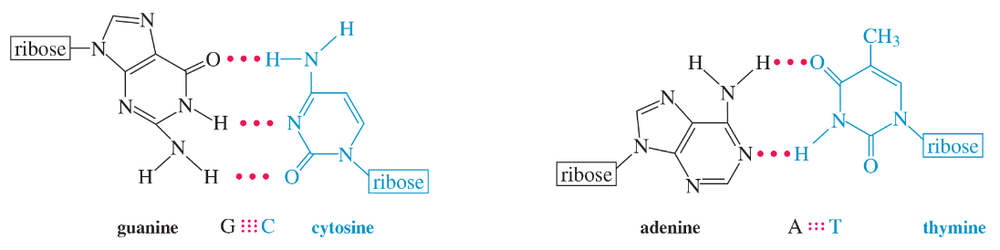
All of the rings of the four heterocyclic bases are aromatic. This is more apparent when the polar resonance forms of the amide groups are drawn, as is done for thymine here. Redraw the hydrogen-bonded guanine-cytosine and adenine-thymine pairs shown in Figure 23-24, using the polar resonance forms of the amides. Show how these forms help to explain why the hydrogen bonds involved in these pairings are particularly strong. Remember that a hydrogen bond arises between an electron-deficient hydrogen atom and an electron-rich pair of nonbonding electrons.
Problem 46
Glucose is the most abundant monosaccharide. From memory, draw glucose in
(a) the Fischer projection of the open chain.
(b) the most stable chair conformation of the most stable pyranose anomer.
(c) the Haworth projection of the most stable pyranose anomer.
Problem 47a
Without referring to the chapter, draw the chair conformations of
(a) β-D-mannopyranose (the C2 epimer of glucose).
Problem 47b
Without referring to the chapter, draw the chair conformations of
(b) α-D-allopyranose (the C3 epimer of glucose).
Problem 47c
Without referring to the chapter, draw the chair conformations of
(c) β-D-galactopyranose (the C4 epimer of glucose).
Problem 47d
Without referring to the chapter, draw the chair conformations of
(d) N-acetylglucosamine, glucose with the C2 oxygen atom replaced by an acetylated amino group.
Problem 48a,b,c
Use Figure 23-3 (the D family of aldoses) to name the following aldoses.
(a) the C2 epimer of D-arabinose
(b) the C3 epimer of D-mannose
(c) the C3 epimer of D-threose