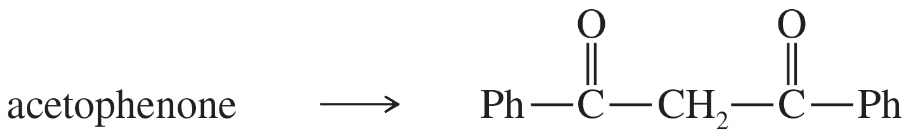Back
BackProblem 1a,b
Phenylacetone can form two different enols.
(a) Show the structures of these enols.
(b) Predict which enol will be present in the larger concentration at equilibrium.
Problem 1c3
Phenylacetone can form two different enols.
(c) Propose mechanisms for the formation of the first enol in base.
Problem 1c4
Phenylacetone can form two different enols.
(c) Propose mechanisms for the formation of the second enol in base.
Problem 1c2
Phenylacetone can form two different enols.
(c) Propose mechanisms for the formation of the second enol in acid.
Problem 1c1
Phenylacetone can form two different enols.
(c) Propose mechanisms for the formation of the first enol in acid.
Problem 2a
Show each step in the mechanism of the acid-catalyzed interconversion of (R)- and (S)-3-methylpentan-2-one.
Problem 2b
When cis-2,4-dimethylcyclohexanone is dissolved in aqueous ethanol containing a trace of NaOH, a mixture of cis and trans isomers results. Propose a mechanism for this isomerization.
Problem 3a,b
Give the important resonance forms for the possible enolate ions of the following:
(a) acetone
(b) cyclopentanone
Problem 3c
Give the important resonance forms for the possible enolate ions of the following:
(c) pentane-2,4-dione
Problem 3d
Give the important resonance forms for the possible enolate ions of the following:
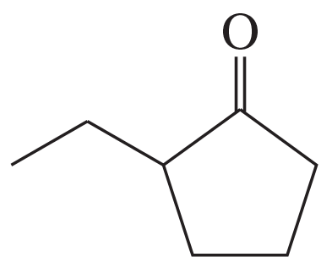
(d)
Problem 3e
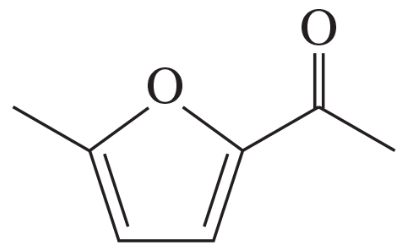
Give the important resonance forms for the possible enolate ions of the following:
(e)
Problem 3f
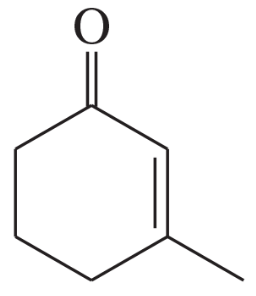
Give the important resonance forms for the possible enolate ions of the following:
(f)
Problem 5
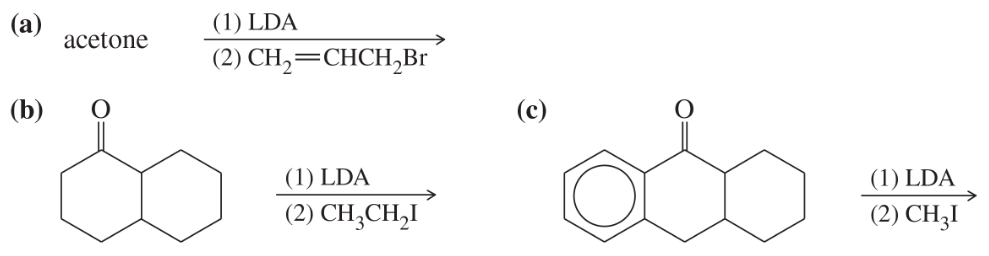
Predict the major products of the following reactions.
Problem 6
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
Problem 7
Without looking back, propose a mechanism for the hydrolysis of this iminium salt to the alkylated ketone. The first step is attack by water, followed by loss of a proton to give a carbinolamine. Protonation on nitrogen allows pyrrolidine to leave, giving the protonated ketone.
Problem 8a
Give the expected products of the following acid-catalyzed reactions.
(a) acetophenone + methylamine
Problem 8b
Give the expected products of the following acid-catalyzed reactions.
(b) acetophenone + dimethylamine
Problem 8c
Give the expected products of the following acid-catalyzed reactions.
(c) cyclohexanone + aniline
Problem 8d
Give the expected products of the following acid-catalyzed reactions.
(d) cyclohexanone + piperidine
Problem 9a,b
Show how you would accomplish each conversion using an enamine synthesis with pyrrolidine as the secondary amine.
(a) cyclopentanone → 2-allylcyclopentanone
(b) pentan-3-one → 2-methyl-1-phenylpentan-3-one
Problem 9c
Show how you would accomplish each conversion using an enamine synthesis with pyrrolidine as the secondary amine.
(c)
Problem 11
Propose a mechanism to show how acetophenone undergoes base-promoted chlorination to give trichloroacetophenone.
Problem 12
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
Problem 13
Predict the products of the following reactions.
(a) cyclopentyl methyl ketone + excess Cl2 + excess NaOH
(b) 1-cyclopentylethanol + excess I2 + excess NaOH
(c) propiophenone + excess Br2 + excess NaOH
Problem 14
Which compounds will give positive iodoform tests?
(a) 1-phenylethanol
(b) pentan-2-one
(c) pentan-2-ol
(d) pentan-3-one
(e) acetone
(f) isopropyl alcohol
Problem 15
Propose a mechanism for the acid-catalyzed bromination of pentan-3-one.
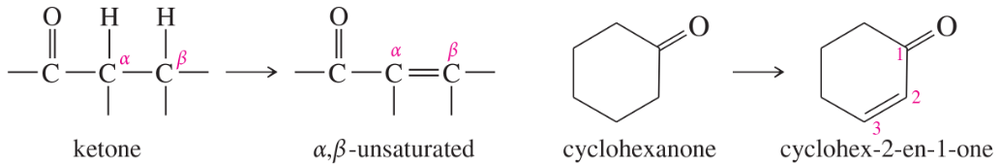
Problem 16
Acid-catalyzed halogenation is synthetically useful for converting ketones to α,β-unsaturated ketones, which are useful in Michael reactions (Section 22-18). Propose a method for converting cyclohexanone to cyclohex-2-en-1-one, an important synthetic starting material.
Problem 17
Show the products of the reactions of these carboxylic acids with PBr3/Br2 before and after hydrolysis.
(a) pentanoic acid
(b) phenylacetic acid
(c) succinic acid
(d) oxalic acid
Problem 18
Propose a mechanism for the aldol condensation of cyclohexanone. Do you expect the equilibrium to favor the reactant or the product?
Problem 19a
Give the expected products for the aldol condensations of (a) propanal.