Back
BackProblem 49
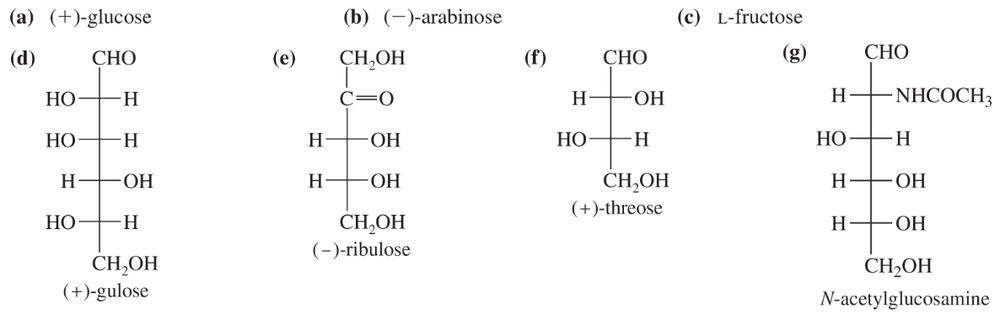
Classify the following monosaccharides. (Examples: D-aldohexose, L-ketotetrose.)
Problem 50
(a) Give the products expected when (+)-glyceraldehyde reacts with HCN.
(b) What is the relationship between the products? How might they be separated?
(c) Are the products optically active? Explain.
Problem 51
The relative configurations of the stereoisomers of tartaric acid were established by the following syntheses:
(1) D-(+)-glyceraldehyde diastereomers A and B (separated)
(2) Hydrolysis of A and B using aqueous Ba(OH)2 gave C and D, respectively.
(3) HNO3 oxidation of C and D gave (-)-tartaric acid and meso-tartaric acid, respectively.
(a) You know the absolute configuration of D-(+)-glyceraldehyde. Use Fischer projections to show the absolute configurations of products A, B, C, and D.
(b) Show the absolute configurations of the three stereoisomers of tartaric acid: (+)-tartaric acid, (-)-tartaric acid, and meso-tartaric acid.
Problem 52b
Predict the products obtained when d-galactose reacts with each reagent.
(b) NaOH, H2O
Problem 52c
Predict the products obtained when d-galactose reacts with each reagent.
(c) CH3OH, H+
Problem 52d
Predict the products obtained when D-galactose reacts with each reagent.
(d) Ag(NH3)2+ –OH
Problem 52e
Predict the products obtained when D-galactose reacts with each reagent.
(e) H2, Ni
Problem 52f
Predict the products obtained when d-galactose reacts with each reagent.
(f) excess Ac2O and pyridine
Problem 52g
Predict the products obtained when d-galactose reacts with each reagent.
(g) excess CH3I, Ag2O
Problem 52h
Predict the products obtained when D-galactose reacts with each reagent.
(h) NaBH4
Problem 52i
Predict the products obtained when D-galactose reacts with each reagent.
(i) Br2, H2O, then H2O2 and Fe2(SO4)3
Problem 52j
Predict the products obtained when D-galactose reacts with each reagent.
(j) (1) KCN/HCN; (2) H2, Pd/BaSO4; (3) H3O+
Problem 52k
Predict the products obtained when D-galactose reacts with each reagent.
(k) excess HIO4
Problem 53
Draw the following sugar derivatives.
(a) methyl β-D-glucopyranoside
(b) 2,3,4,6-tetra-O-methyl-D-mannopyranose
(c) 1,3,6-tri-O-methyl-D-fructofuranose
(d) methyl 2,3,4,6-tetra-O-methyl-β-D-galactopyranoside
Problem 54
Draw the structures (using chair conformations of pyranoses) of the following disaccharides.
(a) 4-O-(α-D-glucopyranosyl)-D-galactopyranose
(b) α-D-fructofuranosyl-β-D-mannopyranoside
(c) 6-O-(β-D-galactopyranosyl)-D-glucopyranose
Problem 55
Erwin Chargaff’s discovery that DNA contains equimolar amounts of guanine and cytosine and also equimolar amounts of adenine and thymine has come to be known as Chargaff’s rule:
G = C and A = T
(a) Does Chargaff’s rule imply that equal amounts of guanine and adenine are present in DNA? That is, does G = A?
(b) Does Chargaff’s rule imply that the sum of the purine residues equals the sum of the pyrimidine residues? That is, does A + G = C + T?
(c) Does Chargaff’s rule apply only to double-stranded DNA, or would it also apply to each individual strand if the double helical strand were separated into its two complementary strands?
Problem 56
Which of the following sugars are reducing sugars? Which ones would undergo mutarotation?
(a) methyl β-D-glucopyranoside
(b) 2,3,4,6-tetra-O-methyl-D-mannopyranose
(c) 1,3,6-tri-O-methyl-D-fructofuranose
(d) methyl 2,3,4,6-tetra-O-methyl-β-D-galactopyranoside
Problem 56a
Which of the following sugars are reducing sugars? Which ones would undergo mutarotation?
(a) 4-O-(α-D-glucopyranosyl)-D-galactopyranose
Problem 56b
Which of the following sugars are reducing sugars? Which ones would undergo mutarotation?
(b) α-D-fructofuranosyl-β-D-mannopyranoside
Problem 56c
Which of the following sugars are reducing sugars? Which ones would undergo mutarotation?
(c) 6-O-(β-D-galactopyranosyl)-D-glucopyranose
Problem 58
An unknown reducing disaccharide is found to be unaffected by invertase enzymes. Treatment with an α-galactosidase cleaves the disaccharide to give one molecule of D-fructose and one molecule of D-galactose. When the disaccharide is treated with excess iodomethane and silver oxide and then hydrolyzed in dilute acid, the products are 2,3,4,6-tetra-O-methylgalactose and 1,3,4-tri-O-methylfructose. Propose a structure for this disaccharide, and give its complete systematic name.
Problem 59a
Which of the D-aldopentoses will give optically active aldaric acids on oxidation with HNO3?
Problem 59b
Which of the D-aldotetroses will give optically active aldaric acids on oxidation with HNO3?
Problem 59c
Sugar X is known to be a D-aldohexose. On oxidation with HNO3, X gives an optically inactive aldaric acid. When X is degraded to an aldopentose, oxidation of the aldopentose gives an optically active aldaric acid. Determine the structure of X.
Problem 59d
Even though sugar X gives an optically inactive aldaric acid, the pentose formed by degradation gives an optically active aldaric acid. Does this finding contradict the principle that optically inactive reagents cannot form optically active products?
Problem 59e
Show what product results if the aldopentose formed from degradation of X is further degraded to an aldotetrose. Does HNO3 oxidize this aldotetrose to an optically active aldaric acid?
Problem 60a
When the gum of the shrub Sterculia setigera is subjected to acidic hydrolysis, one of the water-soluble components of the hydrolysate is found to be tagatose. The following information is known about tagatose:
(1) Molecular formula C6H12O6
(2) Undergoes mutarotation.
(3) Does not react with bromine water.
(4) Reduces Tollens reagent to give D-galactonic acid and D-talonic acid.
(5) Methylation of tagatose (using excess CH3I and Ag2O) followed by acidic hydrolysis gives 1,3,4,5-tetra-O-methyltagatose.
(a) Draw a Fischer projection structure for the open-chain form of tagatose.
Problem 61a-d
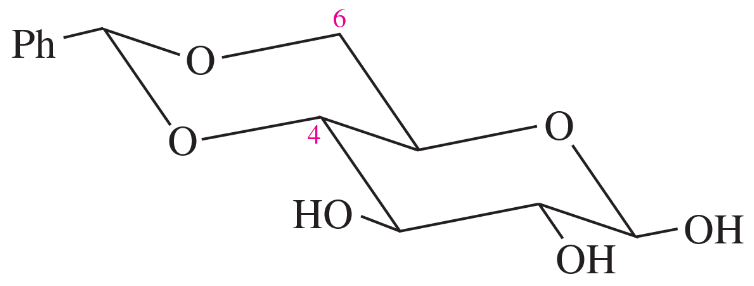
Some protecting groups can block two OH groups of a carbohydrate at the same time. One such group is shown here, protecting the 4-OH and 6-OH groups of β-d-glucose.
(a) What type of functional group is involved in this blocking group?
(b) What did glucose react with to form this protected compound?
(c) When this blocking group is added to glucose, a new chiral center is formed. Where is it? Draw the stereoisomer that has the other configuration at this chiral center. What is the relationship between these two stereoisomers of the protected compound?
(d) Which of the two stereoisomers in part (c) do you expect to be the major product? Why?
Problem 62
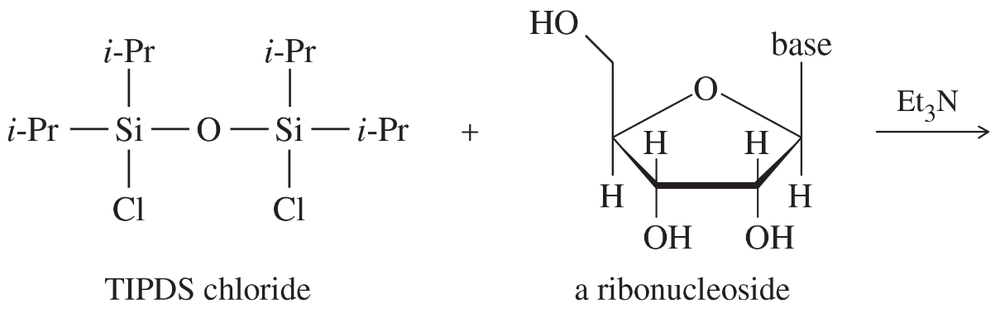
An important protecting group developed specifically for polyhydroxy compounds like nucleosides is the tetraisopropyldisiloxanyl group, abbreviated TIPDS, that can protect two alcohol groups in a molecule.
(a) The TIPDS group is somewhat hindered around the Si atoms by the isopropyl groups. Which OH is more likely to react first with TIPDS chloride? Show the product with the TIPDS group on one oxygen.
(b) Once the TIPDS group is attached at the first oxygen, it reaches around to the next closest oxygen. Show the final product with two oxygens protected.
(c) The unprotected hydroxy group can now undergo reactions without affecting the protected oxygens. Show the product after the protected nucleoside from (b) is treated with tosyl chloride and pyridine, followed by NaBr, ending with deprotection with Bu4NF.
Problem 63a
Draw the structures of the following nucleotides.
(a) guanosine triphosphate (GTP)