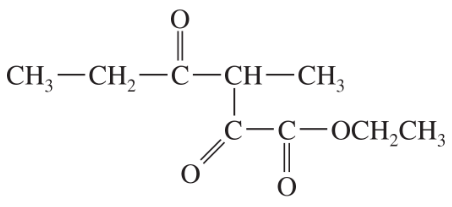
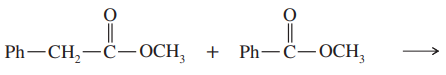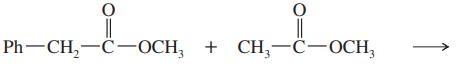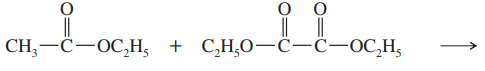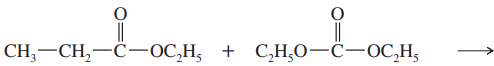Back
BackProblem 38a
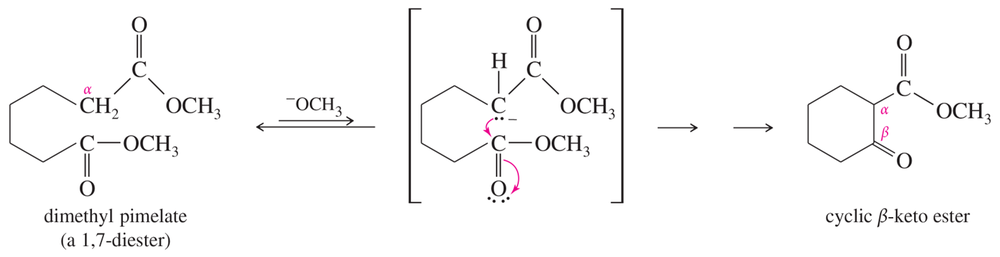
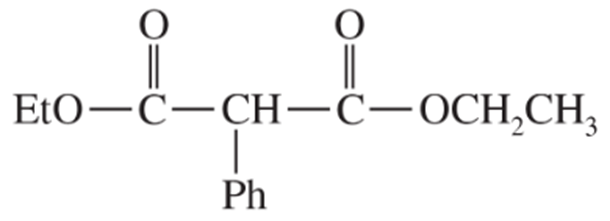
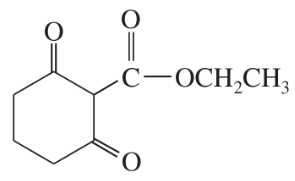
Show what esters would undergo Claisen condensation to give the following β-keto esters.
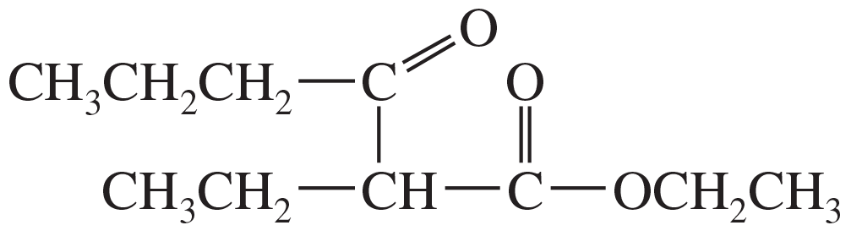
(a)
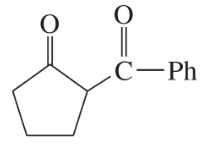
Problem 38b
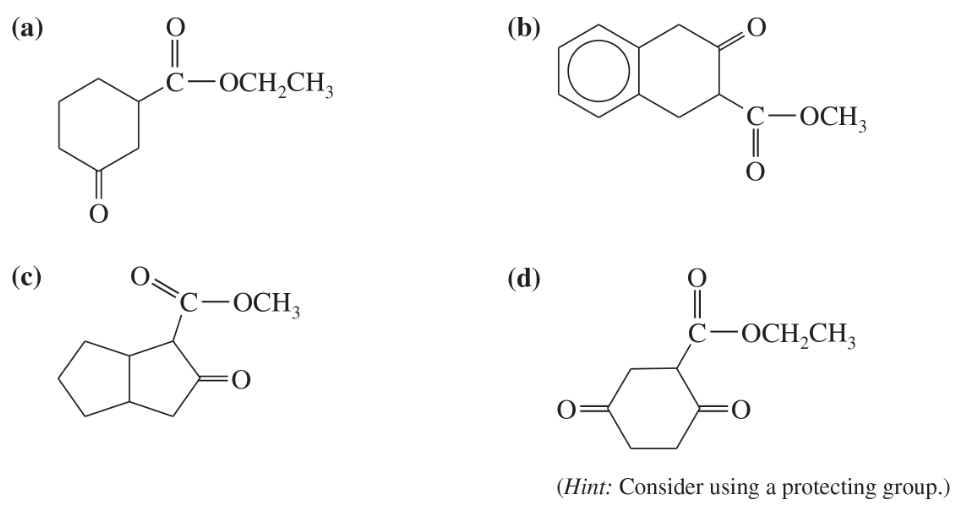
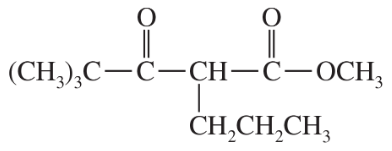
Show what esters would undergo Claisen condensation to give the following β-keto esters.
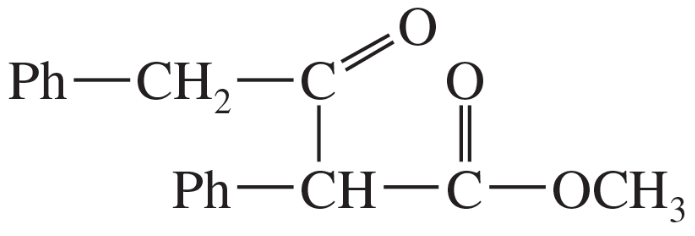
(b)
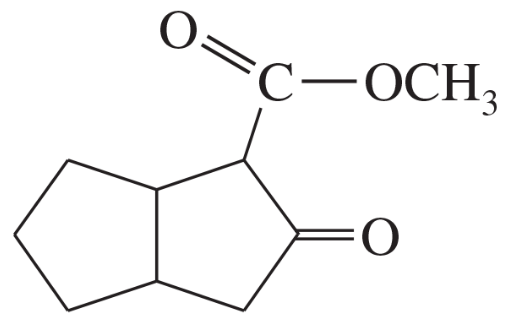
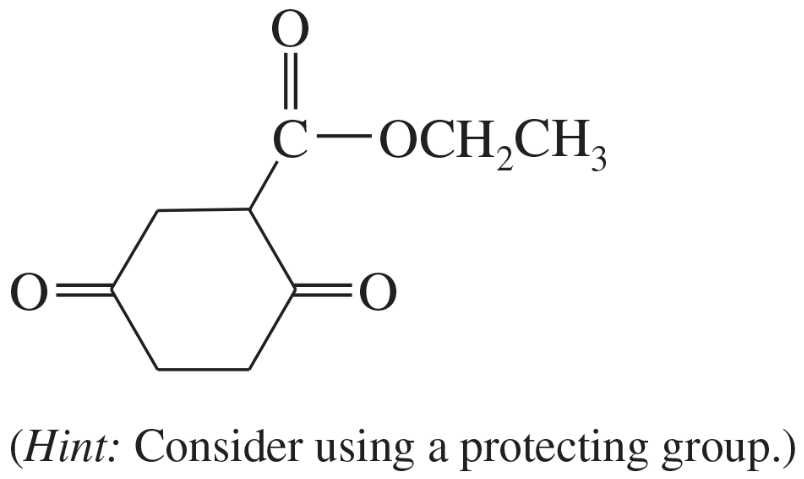
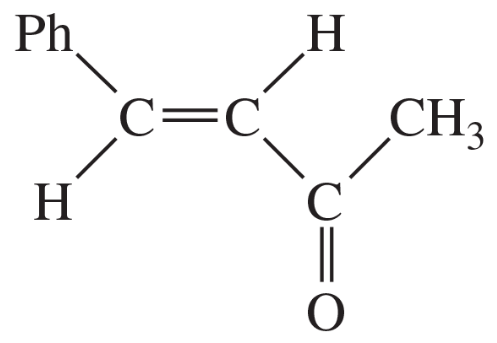
Problem 38c
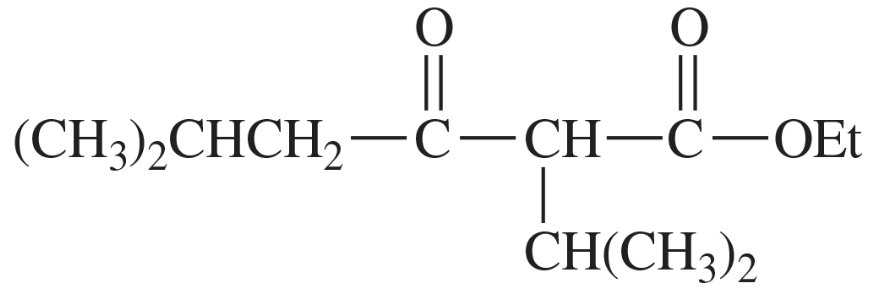
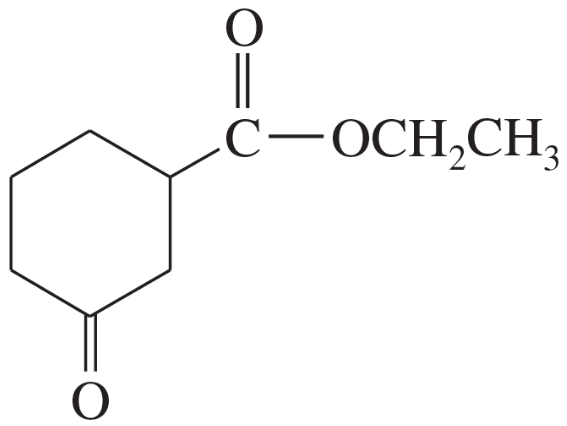
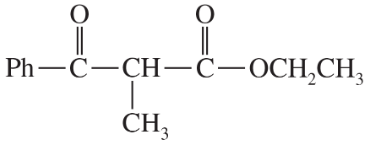
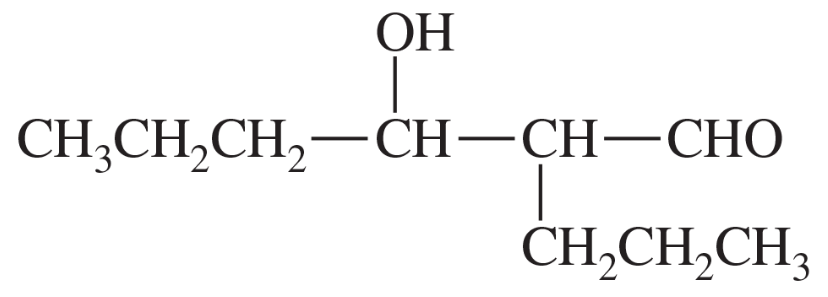
Show what esters would undergo Claisen condensation to give the following β-keto esters.
(c)
Problem 39a
Propose mechanisms for the two Dieckmann condensations just shown.
Problem 39b
Propose mechanisms for the two Dieckmann condensations just shown.
Problem 40
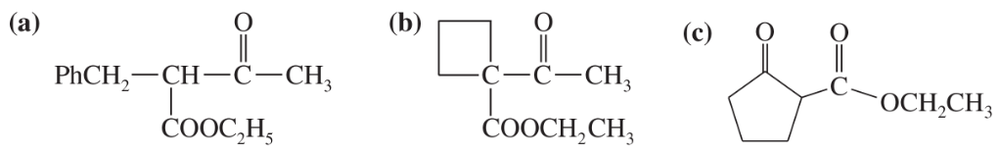
Some (but not all) of the following keto esters can be formed by Dieckmann condensations. Determine which ones are possible, and draw the starting diesters.
Problem 40a,b
Some (but not all) of the following keto esters can be formed by Dieckmann condensations. Determine which ones are possible, and draw the starting diesters.
(a)
(b)
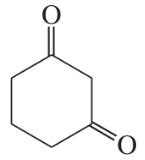
Problem 40c,d
Some (but not all) of the following keto esters can be formed by Dieckmann condensations. Determine which ones are possible, and draw the starting diesters.
(c)
(d)
Problem 41
Propose a mechanism for the crossed Claisen condensation between ethyl acetate and ethyl benzoate.
Problem 42a,b
Predict the products from crossed Claisen condensation of the following pairs of esters. Indicate which combinations are poor choices for crossed Claisen condensations.
(a)
(b)
Problem 42c,d
Predict the products from crossed Claisen condensation of the following pairs of esters. Indicate which combinations are poor choices for crossed Claisen condensations.
(c)
(d)
Problem 43a
Show how crossed Claisen condensations could be used to prepare the following esters.
(a)
Problem 43b
Show how crossed Claisen condensations could be used to prepare the following esters.
(b)
Problem 43c,d
Show how crossed Claisen condensations could be used to prepare the following esters.
(c)
(d)
Problem 44a
Predict the major products of the following crossed Claisen condensations.
(a)
Problem 44b
Predict the major products of the following crossed Claisen condensations.
(b)
Problem 44c
Predict the major products of the following crossed Claisen condensations.
(c)
Problem 45a
Show how Claisen condensations could be used to make the following compounds.
(a)
Problem 45b
Show how Claisen condensations could be used to make the following compounds.
(b)
Problem 45c
Show how Claisen condensations could be used to make the following compounds.
(c)
Problem 45d
Show how Claisen condensations could be used to make the following compounds.
(d)
Problem 46a,b
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base.
(a) ethyl acetoacetate
(b) pentane-2,4-dione
Problem 46c
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base.
(c) ethyl α-cyanoacetate
Problem 46d
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base.
(d) nitroacetone
Problem 47a,b
Show how the following compounds can be made using the malonic ester synthesis.
(a) 3-phenylpropanoic acid
(b) 2-methylpropanoic acid
Problem 47c,d
Show how the following compounds can be made using the malonic ester synthesis.
(c) 4-phenylbutanoic acid
(d) cyclopentanecarboxylic acid
Problem 49
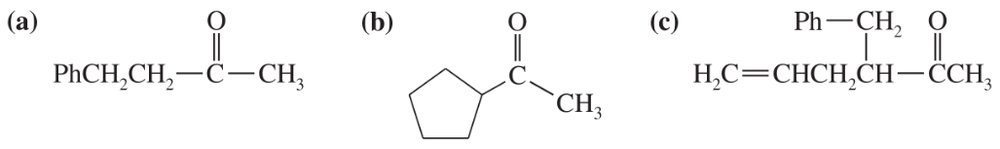
Show the ketones that would result from hydrolysis and decarboxylation of the following β-keto esters.
Problem 50
Show how the following ketones might be synthesized by using the acetoacetic ester synthesis.
Problem 52
In Solved Problem 22-9, the target molecule was synthesized using a Michael addition to form the bond that is β,γ to the upper carbonyl group. Another approach is to use a Michael addition to form the bond that is β,γ to the other (lower) carbonyl group. Show how you would accomplish this alternative synthesis.
Problem 53
Show how cyclohexanone might be converted to the following δ-diketone (Hint: Stork).