Back
BackProblem 19b
Give the expected products for the aldol condensations of (b) phenylacetaldehyde.
Problem 19c
Give the expected products for the aldol condensations of (c) pentan-3-one.
Problem 21
Propose a complete mechanism for the acid-catalyzed aldol condensation of acetone.
Problem 22a,b
Propose a mechanism for the dehydration of diacetone alcohol to mesityl oxide
(a) in acid.
(b) in base.
Problem 24a
Predict the products of aldol condensation, followed by dehydration, of the following ketones and aldehydes.
(a) butyraldehyde
Problem 24b
Predict the products of aldol condensation, followed by dehydration, of the following ketones and aldehydes.
(b) acetophenone
Problem 24c
Predict the products of aldol condensation, followed by dehydration, of the following ketones and aldehydes.
(c) cyclohexanone
Problem 25a
Propose mechanisms for the following base-catalyzed condensations, with dehydration.
(a) 2,2-dimethylpropanal with acetaldehyde
Problem 25b
Propose mechanisms for the following base-catalyzed condensations, with dehydration.
(b) benzaldehyde with propionaldehyde
Problem 28a
Predict the major products of the following base-catalyzed aldol condensations with dehydration.
(a) benzophenone (PhCOPh) + propionaldehyde
Problem 28b
Predict the major products of the following base-catalyzed aldol condensations with dehydration.
(b) 2,2-dimethylpropanal + acetophenone
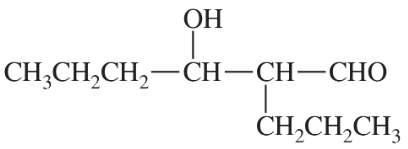
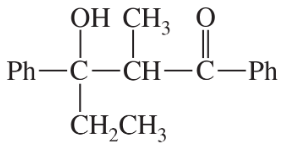
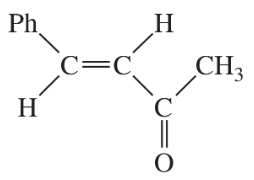
Problem 30
Show how octane-2,7-dione might cyclize to a cycloheptenone. Explain why ring closure to the cycloheptenone is not favored.
Problem 31
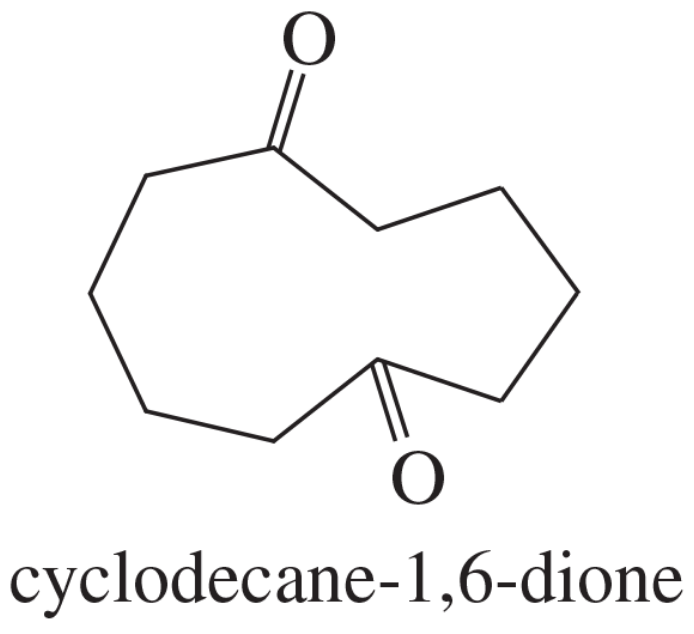
When cyclodecane-1,6-dione is treated with sodium carbonate, the product gives a UV spectrum similar to that of 1-acetyl-2-methylcyclopentene. Propose a structure for the product, and give a mechanism for its formation.
Problem 32a
Show how each compound can be dissected into reagents joined by an aldol condensation, then decide whether the necessary aldol condensation is feasible.
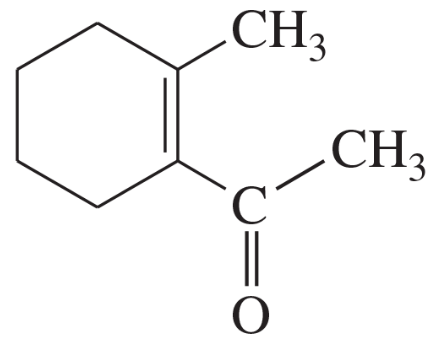
(a)
Problem 32b
Show how each compound can be dissected into reagents joined by an aldol condensation, then decide whether the necessary aldol condensation is feasible.
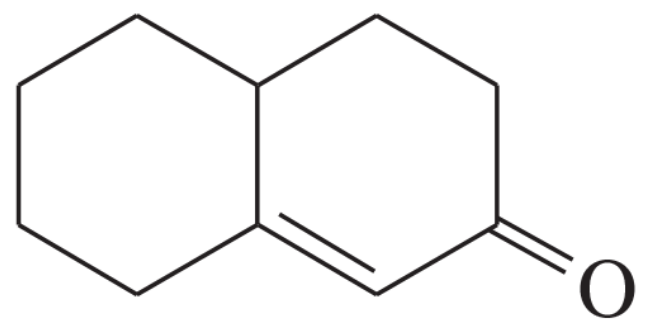
(b)
Problem 32c
Show how each compound can be dissected into reagents joined by an aldol condensation, then decide whether the necessary aldol condensation is feasible.
(c)
Problem 32e
Show how each compound can be dissected into reagents joined by an aldol condensation, then decide whether the necessary aldol condensation is feasible.
(e)
Problem 33a
The following compound results from base-catalyzed aldol cyclization of a 2-substituted cyclohexanone.
(a) Show the diketone that would cyclize to give this product.
Problem 33b
The following compound results from base-catalyzed aldol cyclization of a 2-substituted cyclohexanone.
(b) Propose a mechanism for the cyclization.
Problem 34
Ethoxide is used as the base in the condensation of ethyl acetate to avoid some unwanted side reactions. Show what side reactions would occur if the following bases were used.
(a) sodium methoxide
(b) sodium hydroxide
Problem 35
Esters with only one α hydrogen generally give poor yields in the Claisen condensation. Propose a mechanism for the Claisen condensation of ethyl isobutyrate, and explain why a poor yield is obtained.
Problem 36a,b
Predict the products of self-condensation of the following esters.
(a) methyl propanoate + NaOCH3
(b) ethyl phenylacetate + NaOCH2CH3
Problem 36c
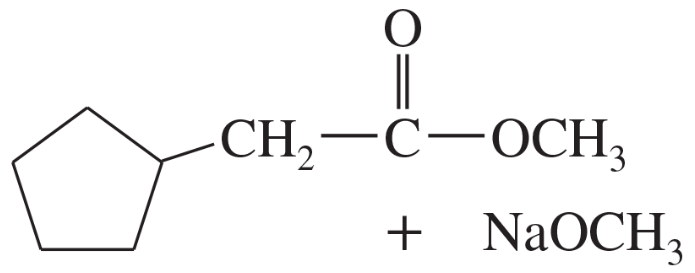
Predict the products of self-condensation of the following esters.
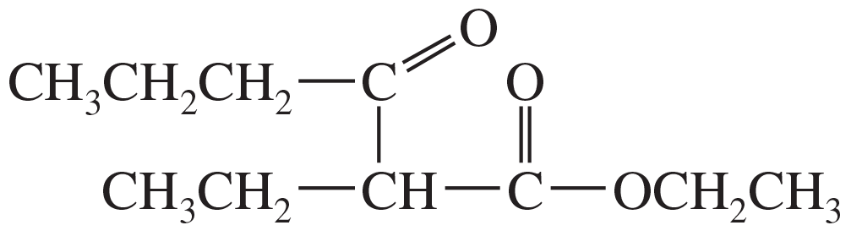
(c)
Problem 36d
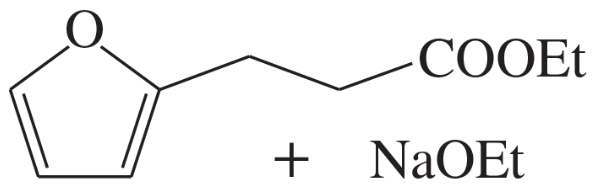
Predict the products of self-condensation of the following esters.
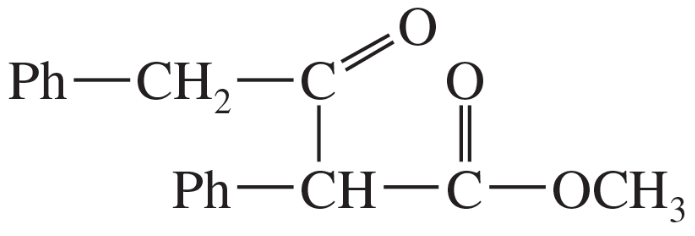
(d)
Problem 37
Propose a mechanism for the self-condensation of methyl 3-phenylpropionate promoted by sodium methoxide.
Problem 38a
Show what esters would undergo Claisen condensation to give the following β-keto esters.
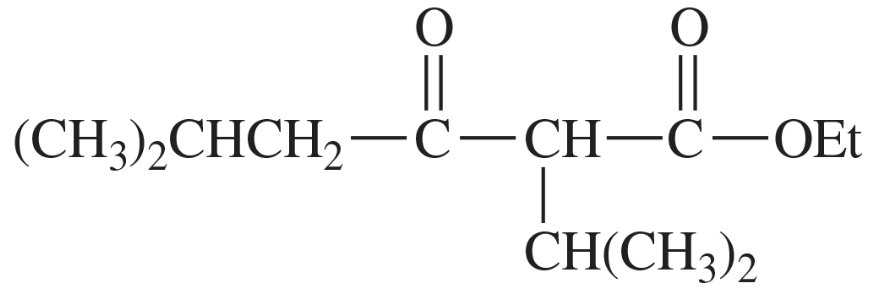
(a)
Problem 38b
Show what esters would undergo Claisen condensation to give the following β-keto esters.
(b)
Problem 38c
Show what esters would undergo Claisen condensation to give the following β-keto esters.
(c)
Problem 39a
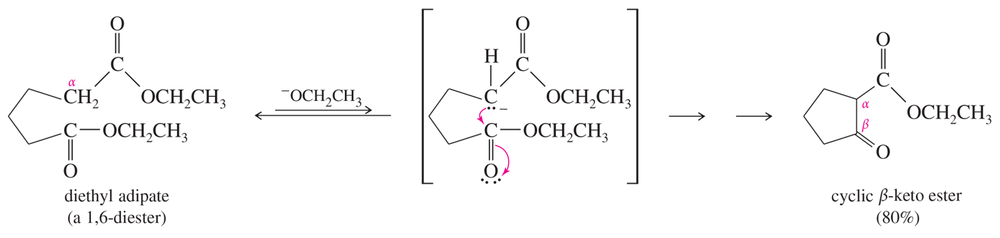
Propose mechanisms for the two Dieckmann condensations just shown.
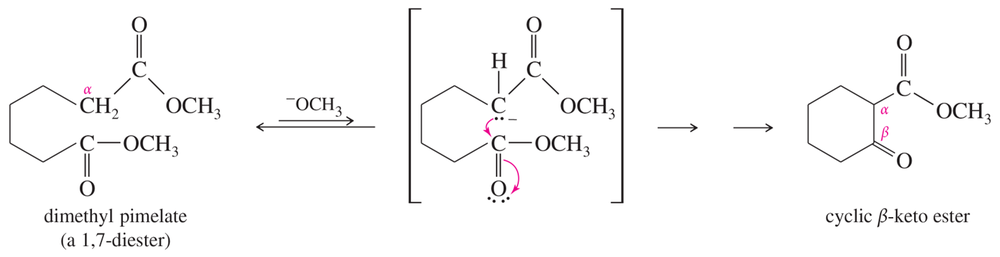
Problem 39b
Propose mechanisms for the two Dieckmann condensations just shown.