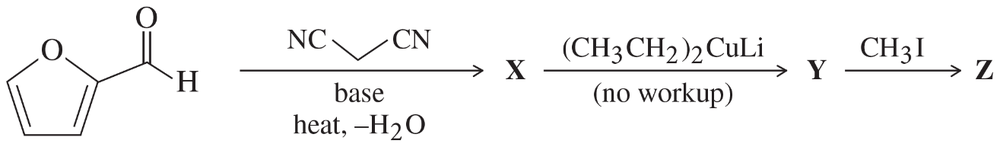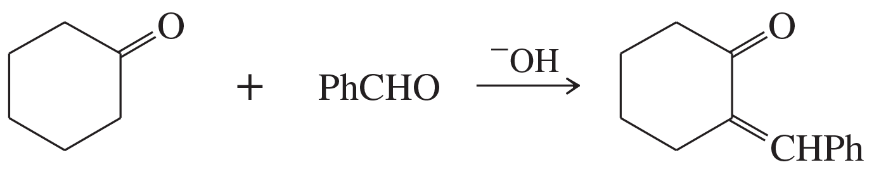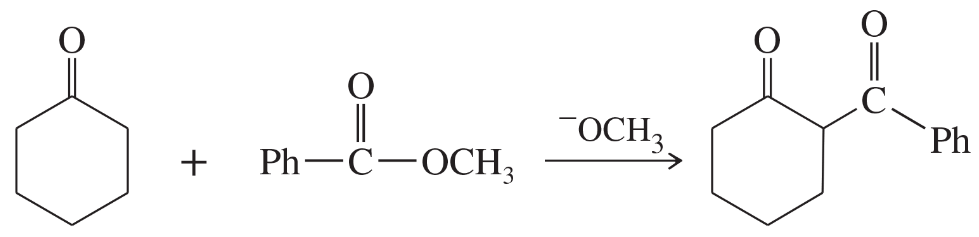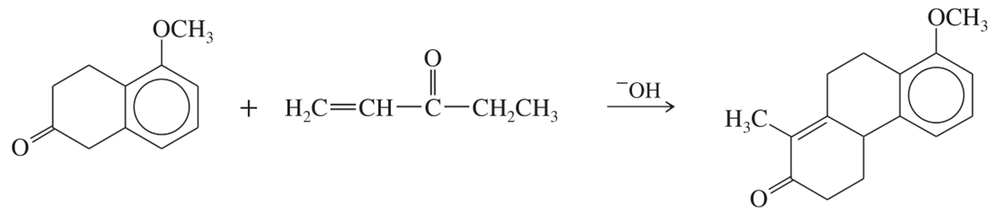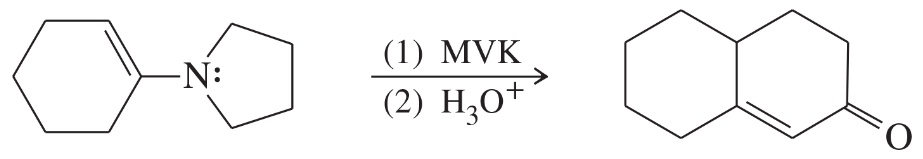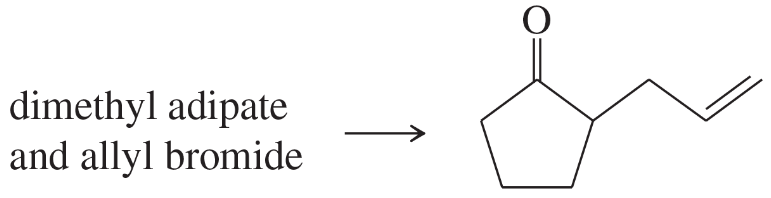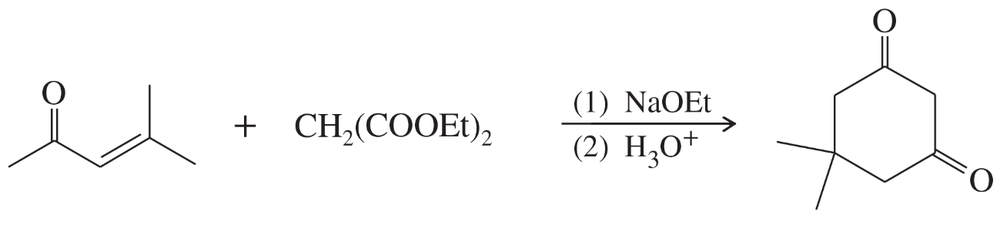Back
BackProblem 75a
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
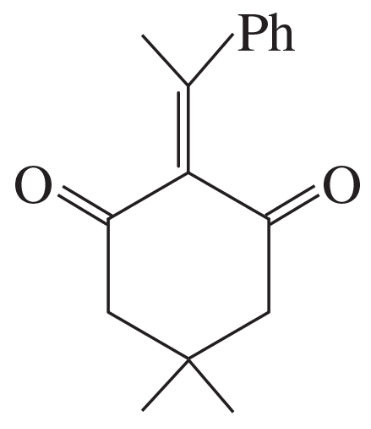
(a)
Problem 75b
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
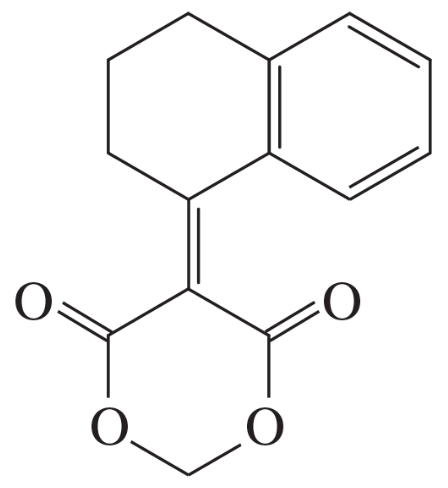
(b)
Problem 75c
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
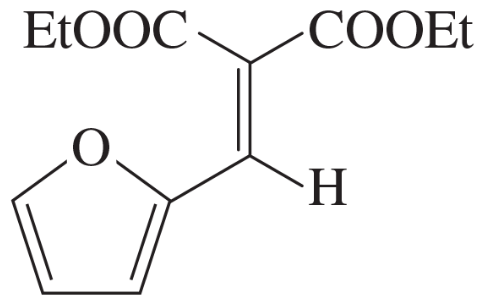
(c)
Problem 75d
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
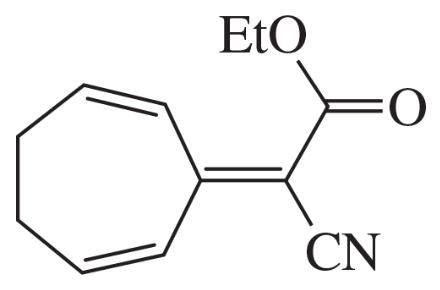
(d)
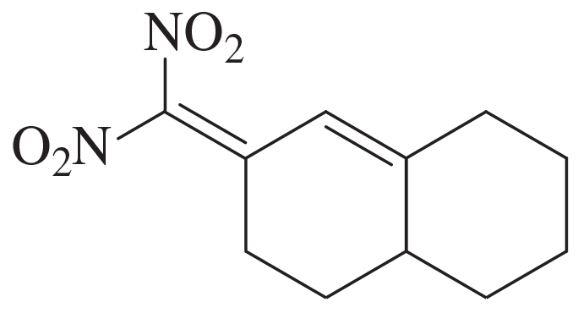
Problem 75e
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
(e)
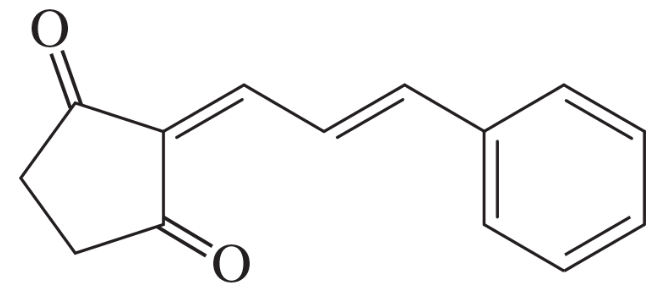
Problem 75f
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an aldehyde or ketone, in the presence of a secondary amine as a basic catalyst, to produce a new C=C. Show the starting materials that made each of these by a Knoevenagel condensation.
(f)
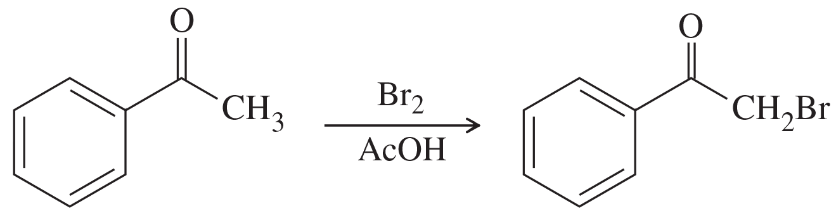
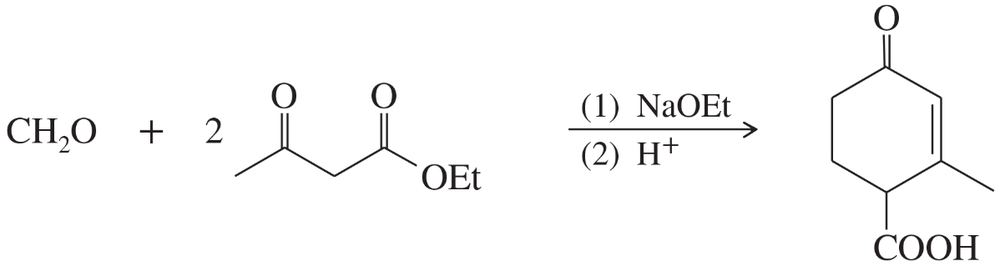
Problem 76
Predict the products from this sequence of reactions.
Problem 77a
Propose mechanisms for the following reactions.
(a)
Problem 77b
Propose mechanisms for the following reactions.
(b)
Problem 77c
Propose mechanisms for the following reactions.
(c)
Problem 77d
Propose mechanisms for the following reactions.
(d)
Problem 77e
Propose mechanisms for the following reactions.
(e)
Problem 78a,b
Write equations showing the expected products of the following enamine alkylation and acylation reactions. Then give the final products expected after hydrolysis of the iminium salts.
(a) pyrrolidine enamine of pentan-3-one + allyl chloride
(b) pyrrolidine enamine of acetophenone + butanoyl chloride
Problem 78c
Write equations showing the expected products of the following enamine alkylation and acylation reactions. Then give the final products expected after hydrolysis of the iminium salts.
(c) piperidine enamine of cyclopentanone + methyl iodide
Problem 78d
Write equations showing the expected products of the following enamine alkylation and acylation reactions. Then give the final products expected after hydrolysis of the iminium salts.
(d) piperidine enamine of cyclopentanone + methyl vinyl ketone
Problem 79a
Show how you would accomplish the following multistep conversions. You may use any additional reagents you need.
(a)
Problem 80a
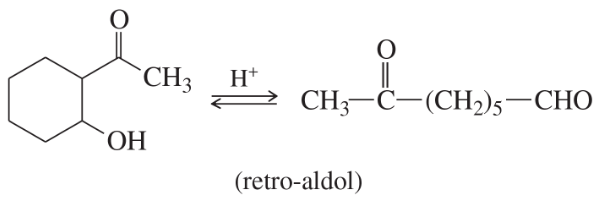
Many of the condensations we have studied are reversible. The reverse reactions are often given the prefix retro-, the Latin word meaning “backward.” Propose mechanisms to account for the following reactions.
(a)
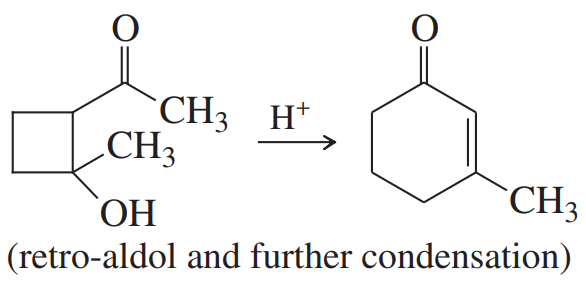
Problem 80b
Many of the condensations we have studied are reversible. The reverse reactions are often given the prefix retro-, the Latin word meaning “backward.” Propose mechanisms to account for the following reactions.
(b)
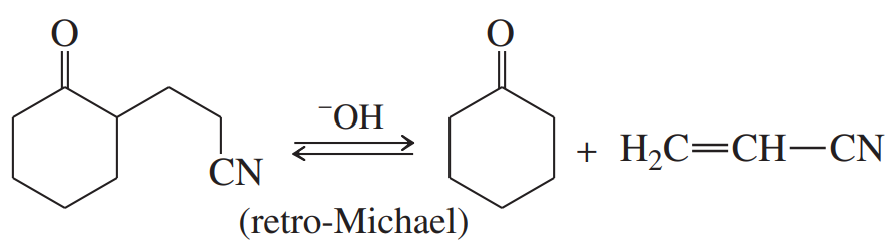
Problem 80c
Many of the condensations we have studied are reversible. The reverse reactions are often given the prefix retro-, the Latin word meaning “backward.” Propose mechanisms to account for the following reactions.
(c)
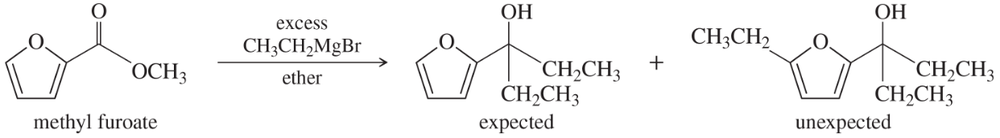
Problem 81
(A true story.) Chemistry lab students added an excess of ethylmagnesium bromide to methyl furoate, expecting the Grignard reagent to add twice and form the tertiary alcohol. After water workup, they found that the product was a mixture of two compounds. One was the expected product having two ethyl groups, but the unexpected product had added three ethyl groups. Propose a mechanism to explain the formation of the unexpected product.
Problem 82a
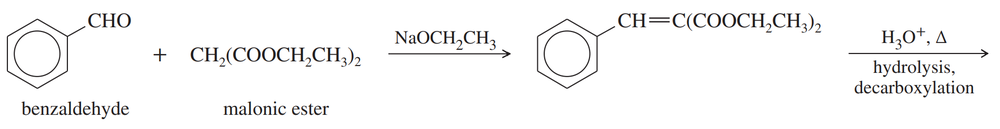
Propose a mechanism for the following reaction.
Problem 82b
Show the structure of the compound that results from hydrolysis and decarboxylation of the product.
Problem 84
Biochemists studying the structure of collagen (a fibrous protein in connective tissue) found cross-links containing α,β-unsaturated aldehydes between protein chains. Show the structures of the side chains that react to form these cross-links, and propose a mechanism for their formation in a weakly acidic solution.
Problem 85a
Show reaction sequences (not detailed mechanisms) that explain these transformations:
(a)
Problem 85b
Show reaction sequences (not detailed mechanisms) that explain these transformations:
(b)