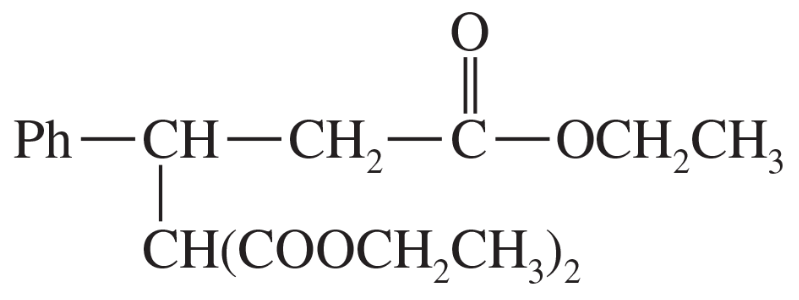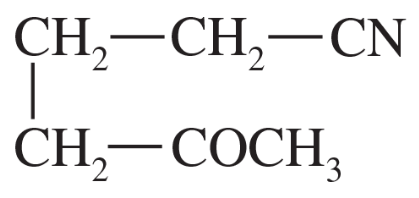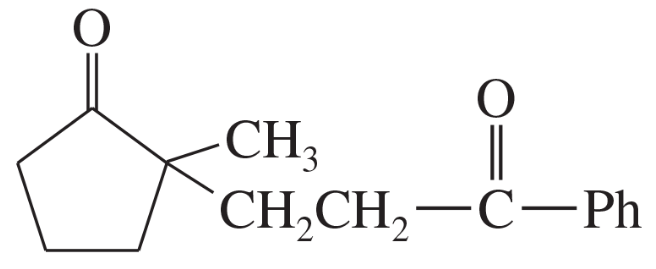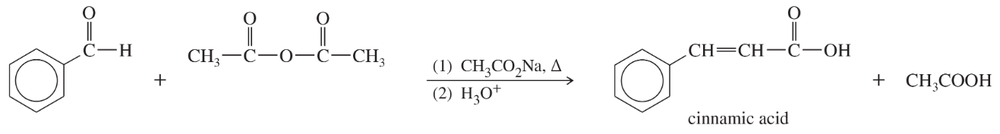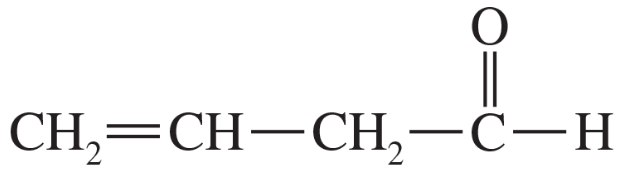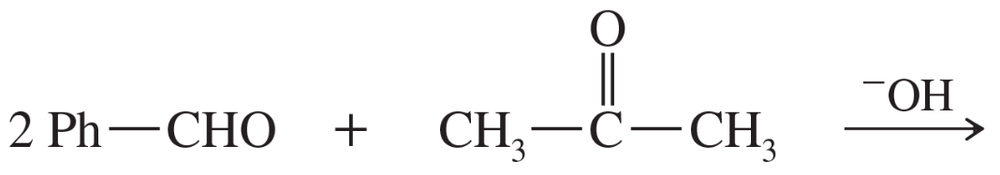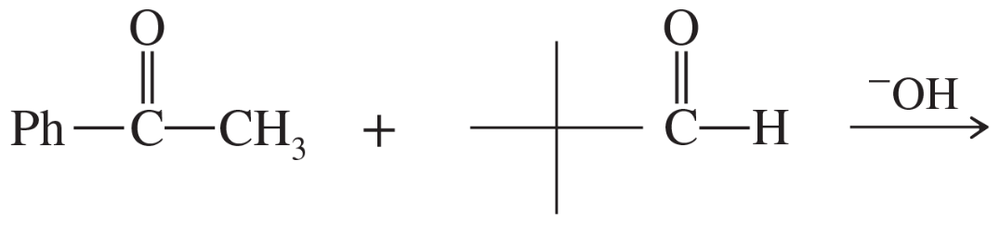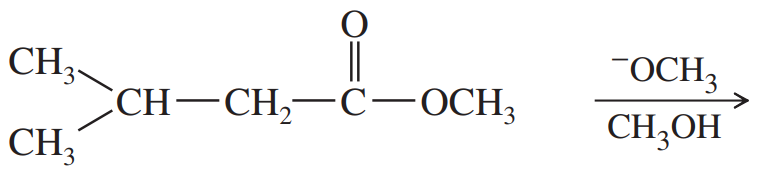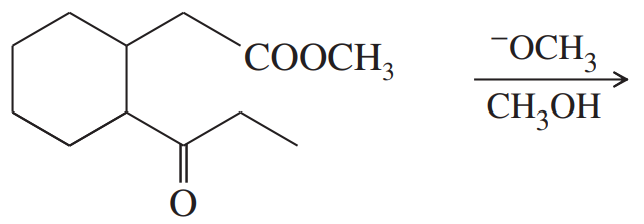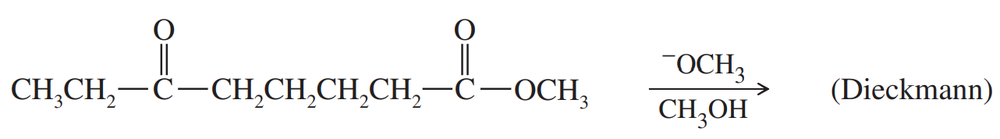Back
BackProblem 54
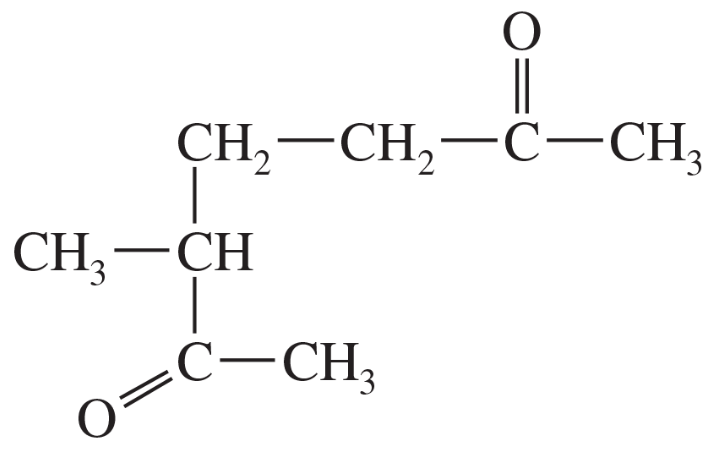
Show how an acetoacetic ester synthesis might be used to form a δ-diketone such as heptane-2,6-dione.
Problem 55a
Propose a mechanism for the conjugate addition of a nucleophile (Nuc:–) to acrylonitrile (H2C=CHCN). Use resonance forms to show how the cyano activate the double bond toward conjugate addition.
Problem 55b
Propose a mechanism for the conjugate addition of a nucleophile (Nuc:–) to acrylonitrile (H2C=CHCN) and to nitroethylene. Use resonance forms to show how the cyano and nitro groups activate the double bond toward conjugate addition.
Problem 56a
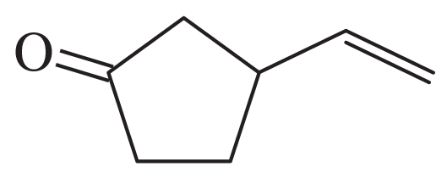
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(a)
Problem 56b
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(b)
Problem 56c
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(c)
Problem 56d
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(d)
Problem 56e
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(e)
Problem 56f
Show how the following products might be synthesized from suitable Michael donors and acceptors.
(f)
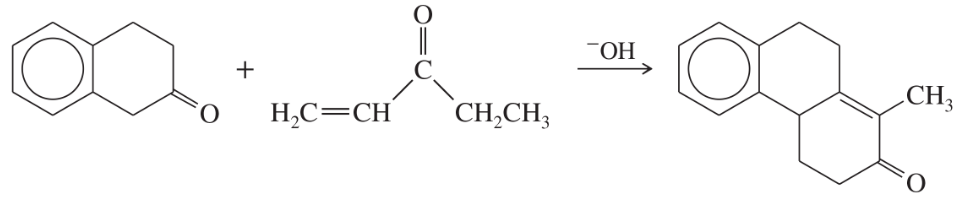
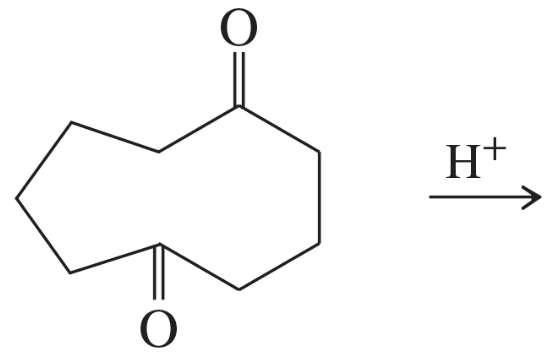
Problem 57
Propose a mechanism for the following reaction.
Problem 58
The base-catalyzed reaction of an aldehyde (having no α hydrogens) with an anhydride is called the Perkin condensation. Propose a mechanism for the following example of the Perkin condensation. (Sodium acetate serves as the base.)
Problem 59a
Show how you would use Robinson annulations to synthesize the following compounds. Work backward, remembering that the cyclohexenone is the new ring and that the double bond of the cyclohexenone is formed by the aldol with dehydration. Take apart the double bond, then see what structures the Michael donor and acceptor must have.
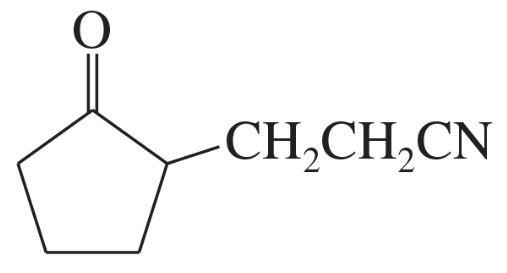
(a)
Problem 59b
Show how you would use Robinson annulations to synthesize the following compounds. Work backward, remembering that the cyclohexenone is the new ring and that the double bond of the cyclohexenone is formed by the aldol with dehydration. Take apart the double bond, then see what structures the Michael donor and acceptor must have.
(b)
Problem 60a,b,c(2)
For each molecule shown below,
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(a)
(b)
(c)
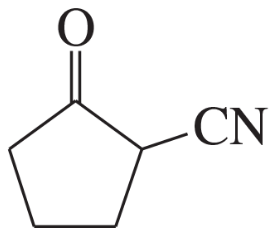
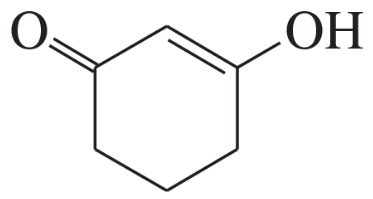
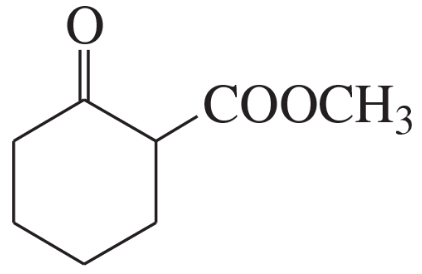
Problem 60a,b,c(1)
For each molecule shown below,
1. indicate the most acidic hydrogens.
(a)
(b)
(c)
Problem 60d
For each molecule shown below,
1. indicate the most acidic hydrogens.
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(d)
Problem 60e
For each molecule shown below,
1. indicate the most acidic hydrogens.
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(e)
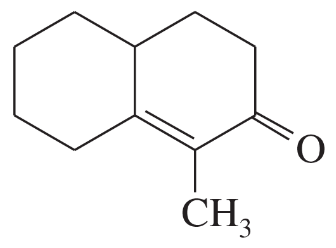
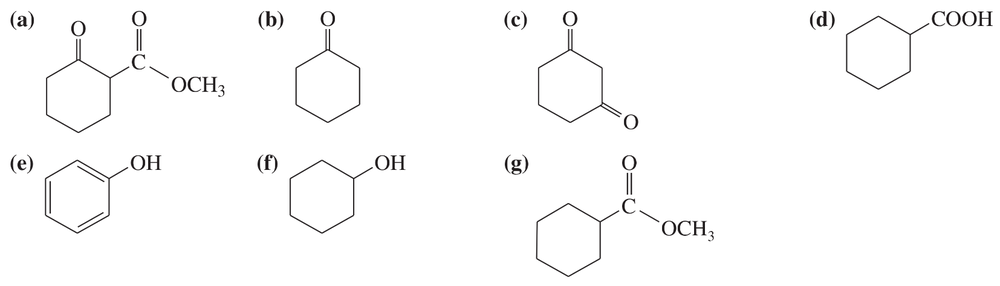
Problem 60g
For each molecule shown below,
1. indicate the most acidic hydrogens.
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(g)
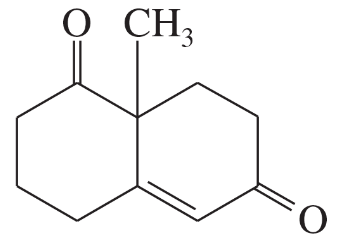
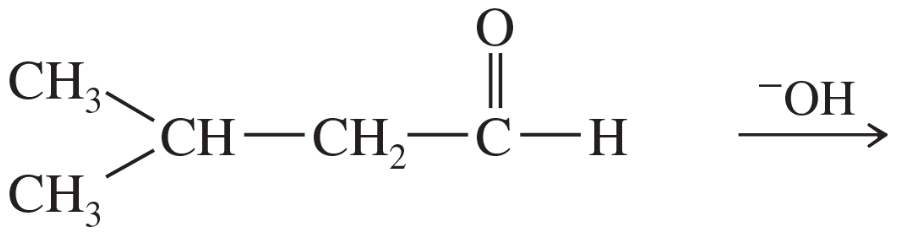
Problem 60h
For each molecule shown below,
1. indicate the most acidic hydrogens.
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(h)
Problem 61(2)
2. Indicate which compounds would be more than 99% deprotonated by a solution of sodium ethoxide in ethanol.
Problem 61(1)
1. Rank the following compounds in order of increasing acidity.
Problem 62a
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(a)
Problem 62b
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(b)
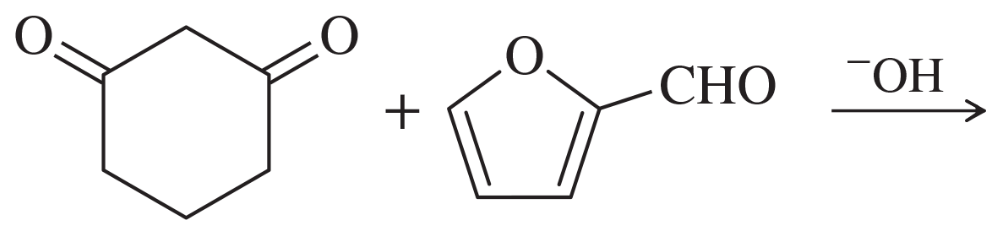
Problem 62c
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(c)
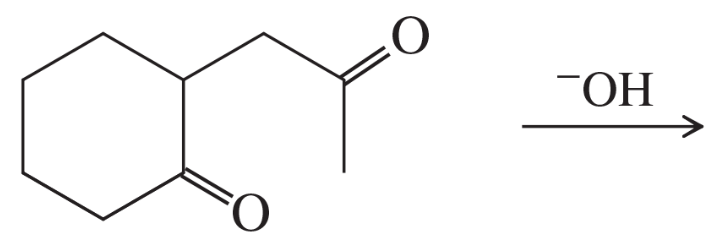
Problem 62d
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(d)
Problem 62e
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(e)
Problem 62f
Predict the products of the following aldol condensations. Show the products both before and after dehydration.
(f)
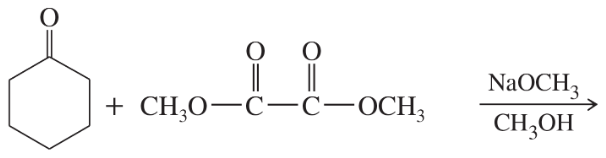
Problem 63a,b
Predict the products of the following Claisen condensations.
(a)
(b)
Problem 63c
Predict the products of the following Claisen condensations.
(c)
Problem 63d
Predict the products of the following Claisen condensations.
(d)