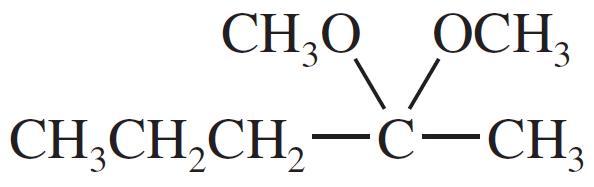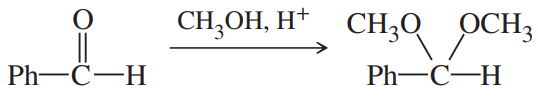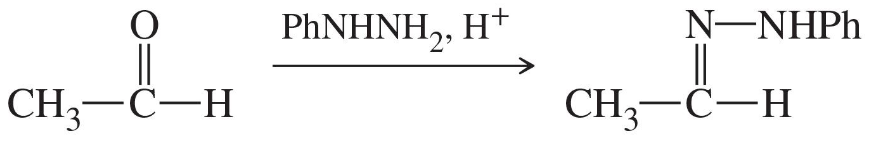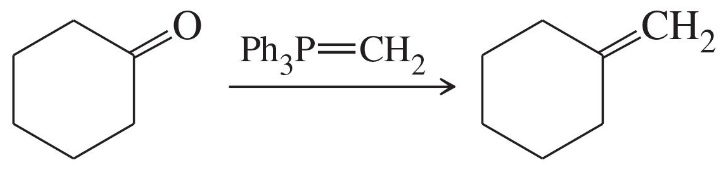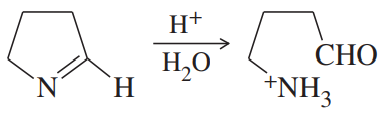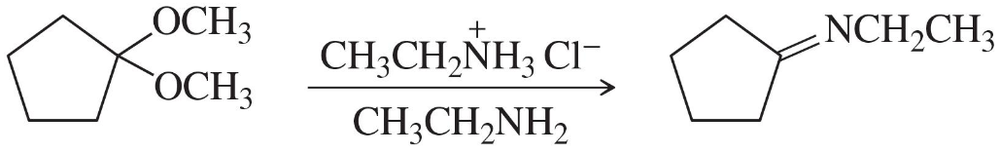Back
BackProblem 41c
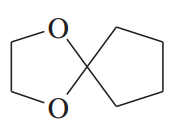
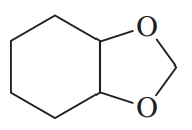
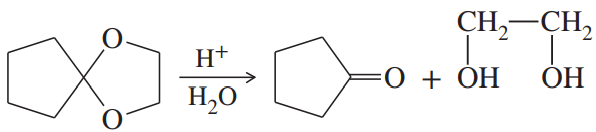
Acetals can serve as protecting groups for 1,2-diols, as well as for aldehydes and ketones. When the acetal is formed from acetone plus the diol, the acetal is called an acetonide. Show the acetonides formed from these diols with acetone under acid catalysis.
Problem 45b
The following compounds undergo McLafferty rearrangement in the mass spectrometer. Predict the masses of the resulting charged fragments.
(b) 3-methylhexan-2-one
Problem 47a
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(a)
Problem 47b
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
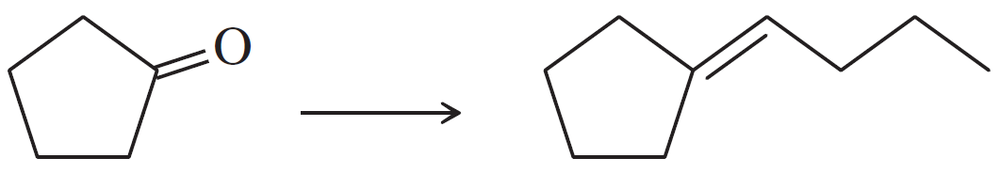
(b)
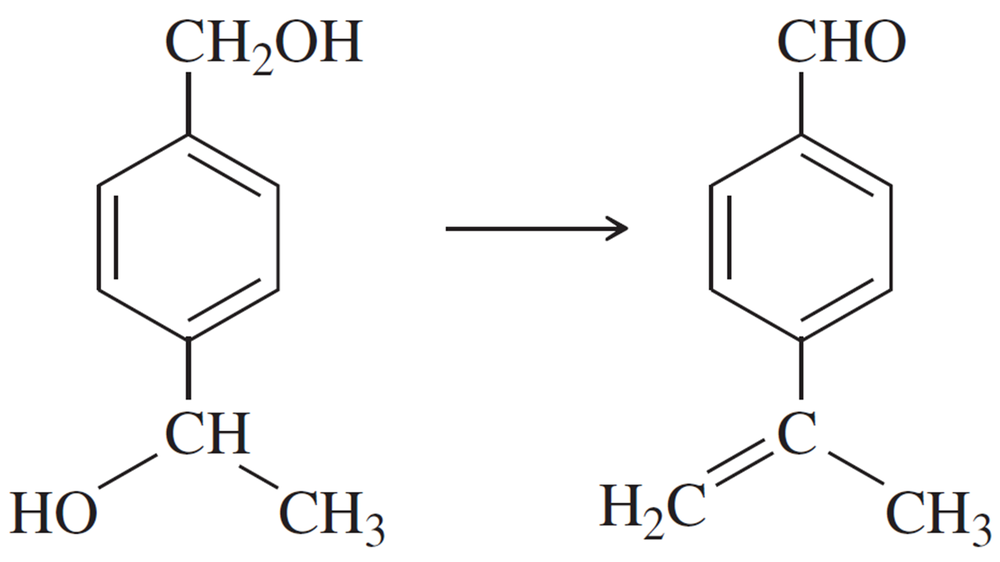
Problem 47c
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
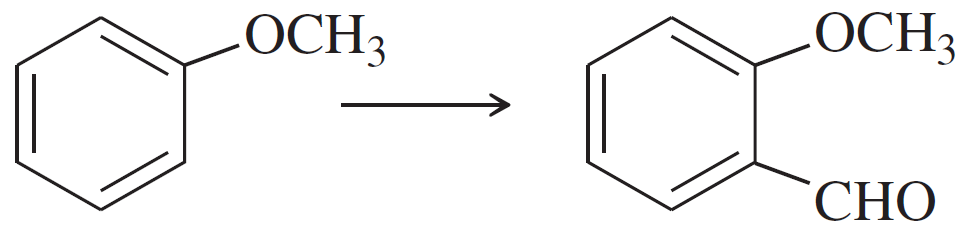
(c)
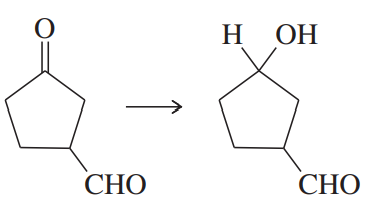
Problem 47d
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
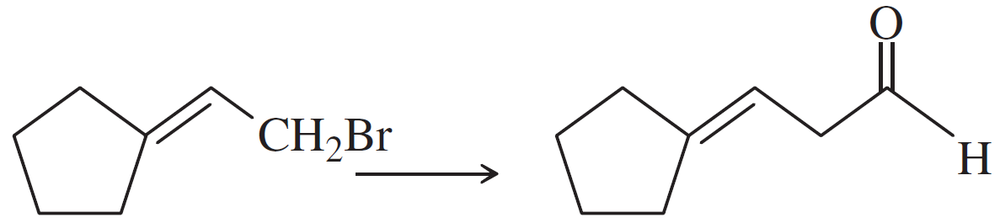
(d)
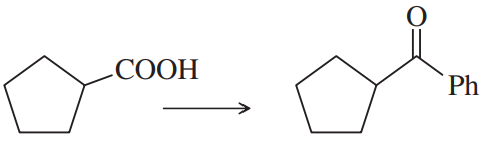
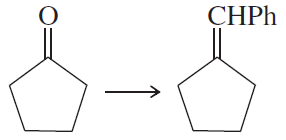
Problem 47e
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(e)
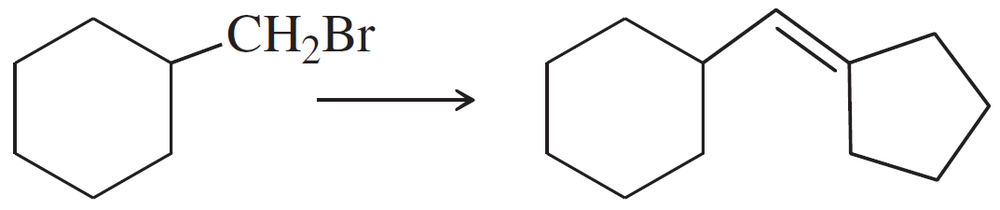
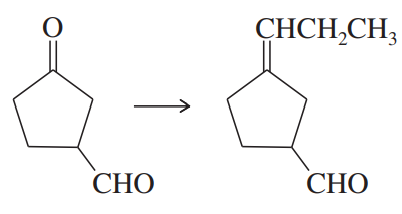
Problem 47f
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(f)
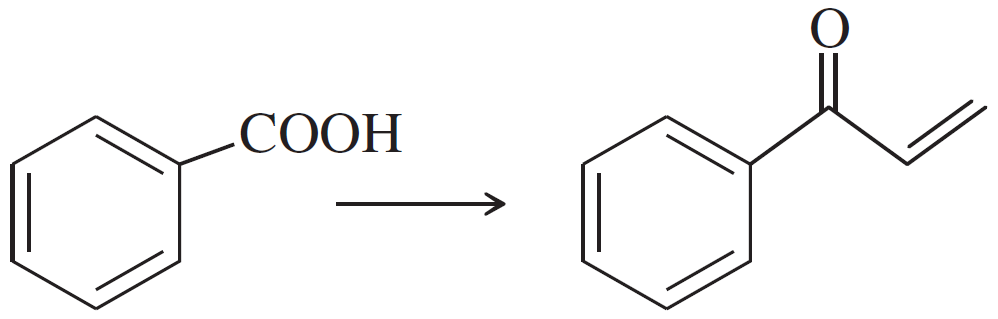
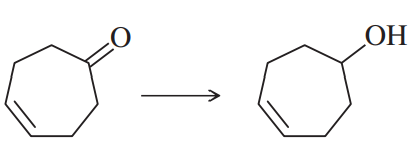
Problem 47g
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(g)
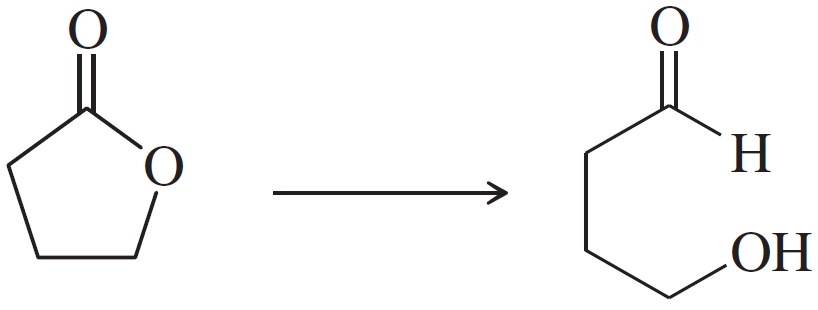
Problem 47h
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(h)
Problem 49a
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(a)
Problem 49b
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(b)
Problem 49c
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(c)
Problem 49d
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
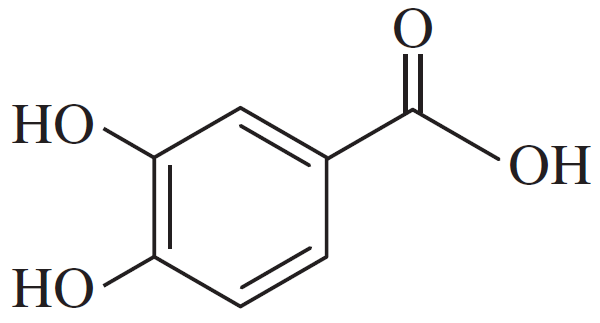
(d)
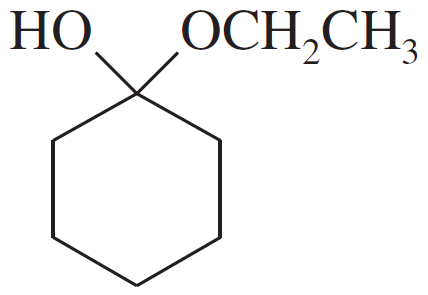
Problem 49e
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(e)
Problem 49g
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(g)
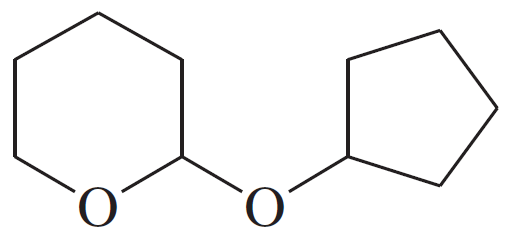
Problem 49h
For each compound,
1. name the functional group.
2. show what compound(s) result from complete hydrolysis.
(h)
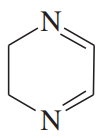
Problem 50a
Propose mechanisms for the following reactions.
(a)
Problem 50b
Propose mechanisms for the following reactions.
(b)
Problem 50c
Propose mechanisms for the following reactions.
(c)
Problem 50d
Propose mechanisms for the following reactions.
(d)
Problem 50e
Propose mechanisms for the following reactions.
(e)
Problem 50f
Propose mechanisms for the following reactions.
(f)
Problem 51a
Show how you would accomplish the following syntheses efficiently and in good yield. You may use any necessary reagents.
(a) acetaldehyde → lactic acid, CH3CH(OH)COOH
Problem 51b
Show how you would accomplish the following syntheses efficiently and in good yield. You may use any necessary reagents.
(b)
Problem 51d
Show how you would accomplish the following synthetic conversions efficiently and in good yield. You may use any necessary additional reagents and solvents.
(d)
Problem 51e
Show how you would accomplish the following syntheses efficiently and in good yield. You may use any necessary reagents.
(e)
Problem 51g
Show how you would accomplish the following syntheses efficiently and in good yield. You may use any necessary reagents.
(g)
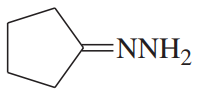
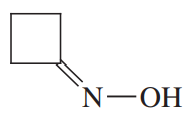
Problem 52a
Show how you would synthesize the following derivatives from appropriate carbonyl compounds.
(a)
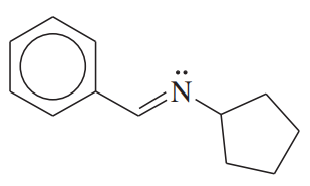
Problem 52b
Show how you would synthesize the following derivatives from appropriate carbonyl compounds.
(b)