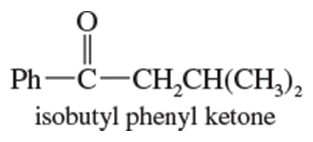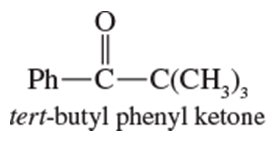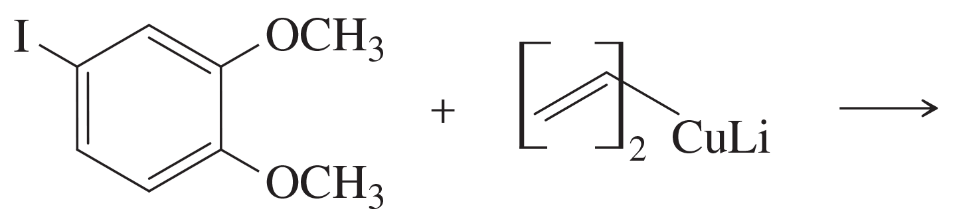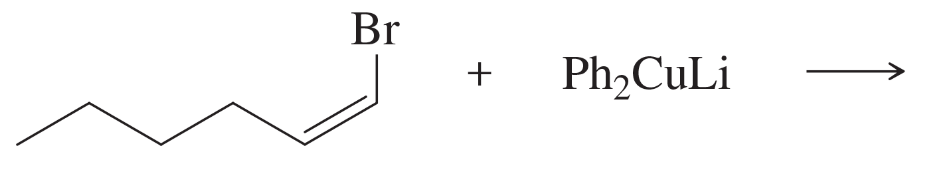Back
BackProblem 19a
Show how you would synthesize the following aromatic derivatives from benzene.
a. p-tert-butylnitrobenzene
Problem 19b
Show how you would synthesize the following aromatic derivatives from benzene.
b. p-toluenesulfonic acid
Problem 19c
Show how you would synthesize the following aromatic derivatives from benzene.
c. p-chlorotoluene
Problem 20a
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
a.
Problem 20b
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
b.
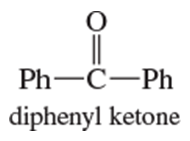
Problem 20c
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
c.
Problem 20f
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
f. 1-phenyl-2,2-dimethylpropane
Problem 20g
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
g. n-butylbenzene
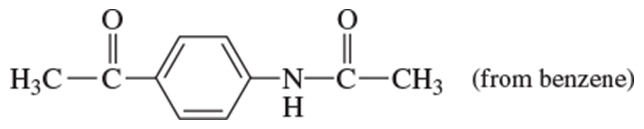
Problem 20h
Show how you would use the Friedel–Crafts acylation, Clemmensen reduction, and/or Gatterman–Koch synthesis to prepare the following compounds:
h.
Problem 22
Propose a mechanism that shows why p-chlorotoluene reacts with sodium hydroxide at 350 °C to give a mixture of p-cresol and m-cresol.
Problem 23a,b
Propose mechanisms and show the expected products of the following reactions.
(a) 2,4-dinitrochlorobenzene + sodium methoxide (NaOCH3)
(b) 2,4-dimethylchlorobenzene + sodium hydroxide, 350 °C
Problem 23c,d
Propose mechanisms and show the expected products of the following reactions.
(c) p-nitrobromobenzene + methylamine (CH3–NH2)
(d) 2,4-dinitrochlorobenzene + excess hydrazine (H2N–NH2)
Problem 24
The highly reactive triple bond of benzyne is a powerful dienophile. Predict the product of the Diels–Alder reaction of benzyne (from chlorobenzene and NaOH, heated) with cyclopentadiene.
Problem 25
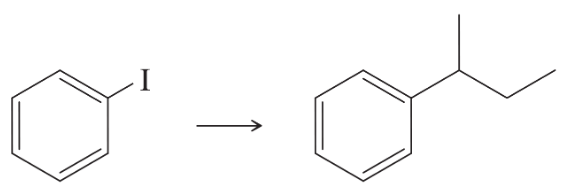
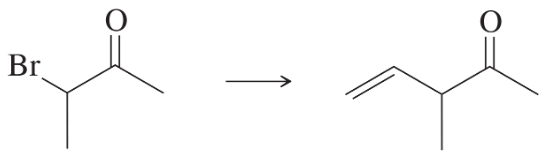
What products would you expect from the following reactions?
(a)
(b)
Problem 26
What organocuprate reagent would you use for the following substitutions?
(a)
(b)
Problem 27a
What products would you expect from the following reactions?
(a)
Problem 27b
What products would you expect from the following reactions?
(b)
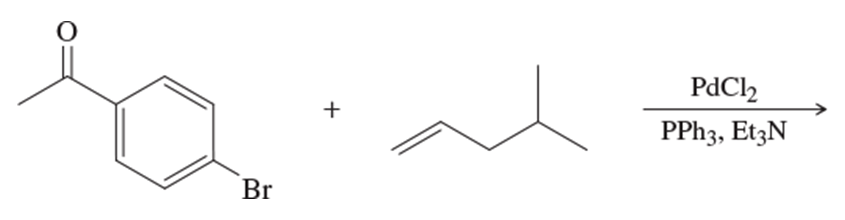
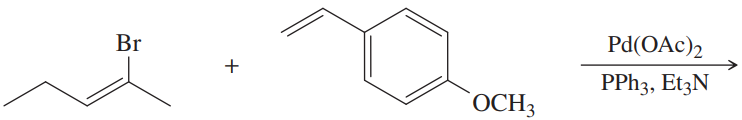
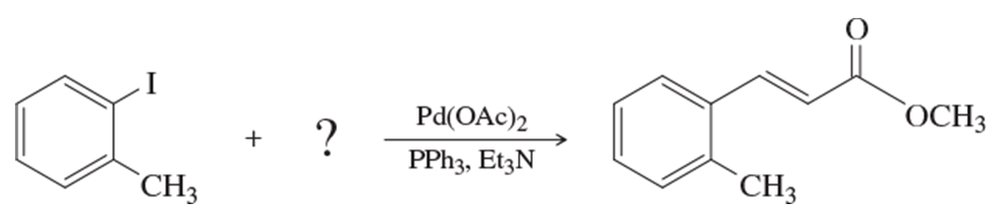
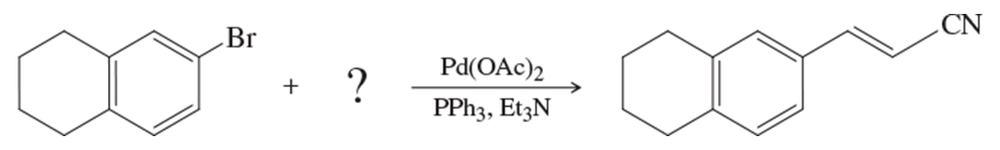
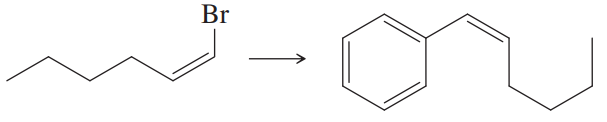
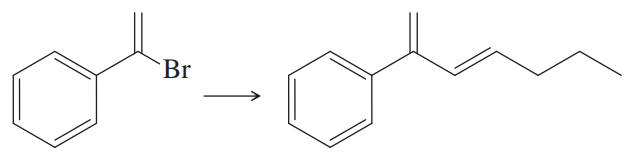
Problem 28
What substituted alkene would you use in the Heck reaction to make the following products?
(a)
(b)
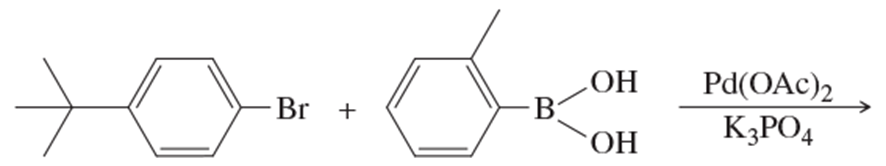
Problem 29a
What products would you expect from the following Suzuki coupling reactions?
(a)
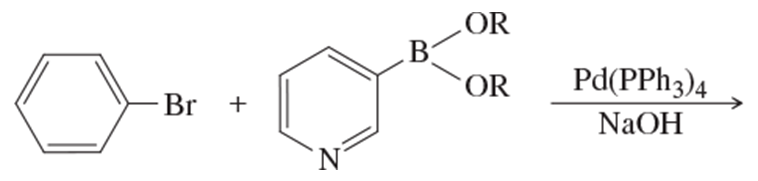
Problem 29b
What products would you expect from the following Suzuki coupling reactions?
(b)
Problem 30a
Show how you would use Suzuki reactions to synthesize these products from the indicated starting materials. You may use any additional reagents you need.
(a)
Problem 30b
Show how you would use Suzuki reactions to synthesize these products from the indicated starting materials. You may use any additional reagents you need.
(b)
Problem 31a
Propose mechanisms for the Birch reduction of benzoic acid. Show why the observed orientation of reduction is favored in each case.
Problem 31b
Propose mechanisms for the Birch reduction of anisole. Show why the observed orientation of reduction is favored in each case.
Problem 32a
Predict the major products of the following reactions.
(a) toluene + excess Cl2 (heat, pressure)
Problem 32b
Predict the major products of the following reactions.
(b) benzamide (PhCONH2) + Na (liquid NH3, CH3CH2OH)
Problem 32c
Predict the major products of the following reactions.
(c) o-xylene + H2 (1000 psi, 100 °C, Rh catalyst)
Problem 32d,e
Predict the major products of the following reactions.
(d) p-xylene + Na (liquid NH3, CH3CH2OH)
(e)
Problem 33
Predict the major products of treating the following compounds with hot, concentrated potassium permanganate, followed by acidification with dilute HCl.
(a) isopropylbenzene
(b) p-xylene
(c)
Problem 34
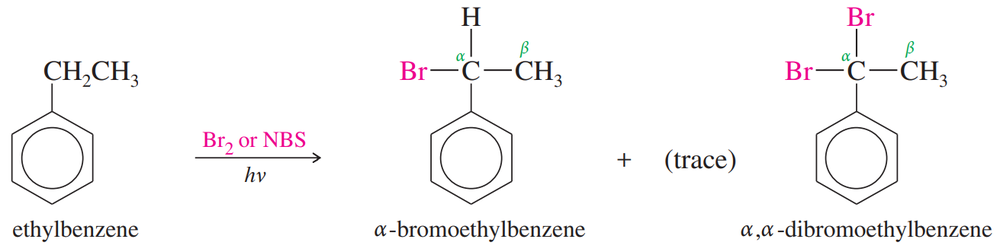
Propose a mechanism for the bromination of ethylbenzene shown below.