Back
BackProblem 72
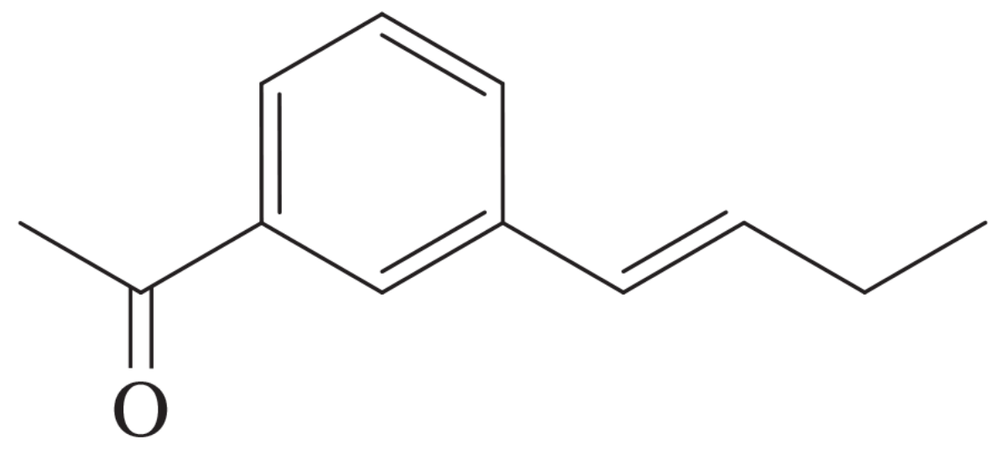
Starting with benzene and any other reagents you need, show how you would synthesize the compound shown here. (Hint: Consider a Pd-catalyzed coupling for the final step.)
Problem 74
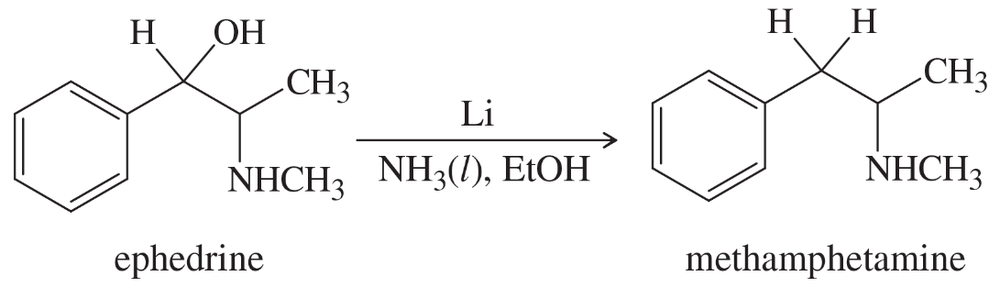
A common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of lithium metal. The Birch reduction usually reduces the aromatic ring (Section 17-14C), but in this case it eliminates the hydroxy group of ephedrine to give methamphetamine. Propose a mechanism, similar to that for the Birch reduction, to explain this unusual course of the reaction.
Problem 75
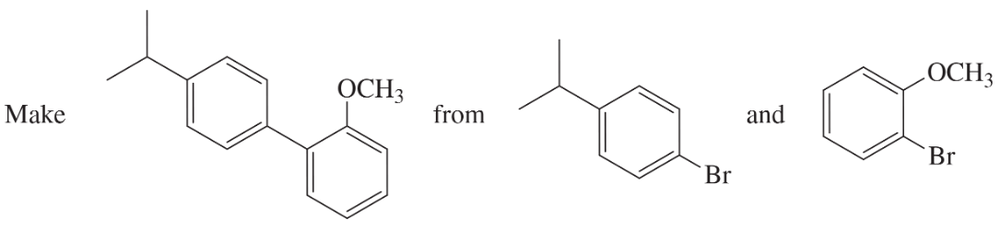
Show how you would use a Suzuki reaction to synthesize the following biaryl compound. As starting materials, you may use the two indicated compounds, plus any additional reagents you need.
Problem 76
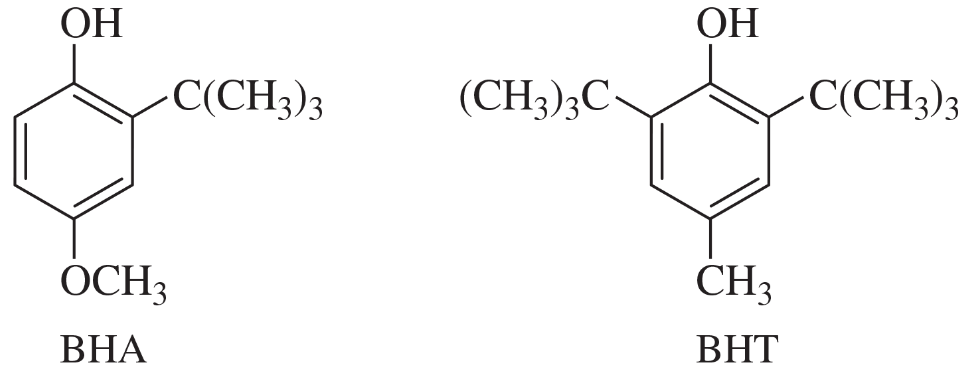
The antioxidants BHA and BHT are commonly used as food preservatives. Show how BHA and BHT can be made from phenol and hydroquinone
Problem 78a
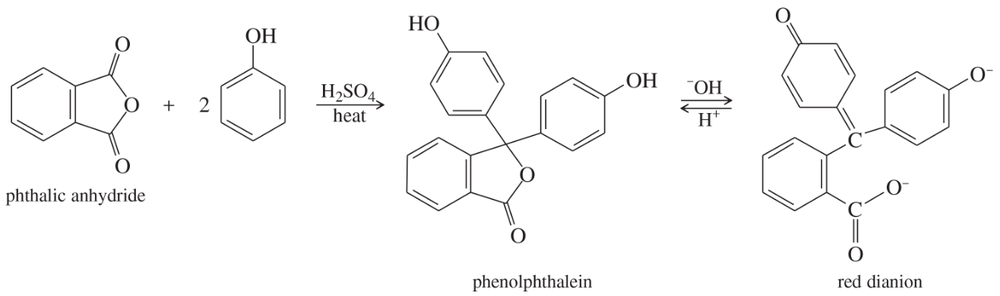
Phenolphthalein, a common nonprescription laxative, is also an acid–base indicator that is colorless in acid and red in base. Phenolphthalein is synthesized by the acid-catalyzed reaction of phthalic anhydride with 2 equivalents of phenol.
a. Propose a mechanism for the synthesis of phenolphthalein.
Problem 78b,c
Phenolphthalein, a common nonprescription laxative, is also an acid–base indicator that is colorless in acid and red in base. Phenolphthalein is synthesized by the acid-catalyzed reaction of phthalic anhydride with 2 equivalents of phenol.
b. Propose a mechanism for the conversion of phenolphthalein to its red dianion in base.
c. Use resonance structures to show that the two phenolic oxygen atoms are equivalent (each with half a negative charge) in the red phenolphthalein dianion.