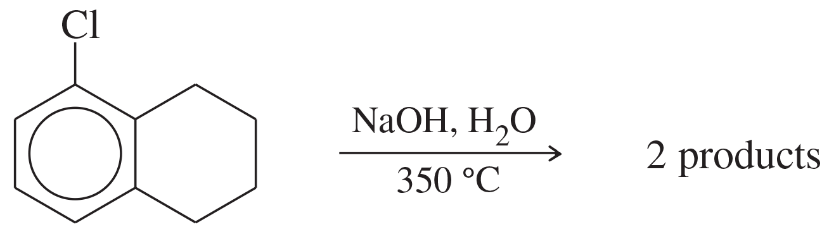Back
BackProblem 53b
Predict the major products of the following reactions.
(b) phenol + tert-butyl chloride + AlCl3
Problem 53c
Predict the major products of the following reactions.
(c) nitrobenzene + fuming sulfuric acid
Problem 53d
Predict the major products of the following reactions.
(d) nitrobenzene + acetyl chloride + AlCl3
Problem 53e
Predict the major products of the following reactions.
(e) p-methylanisole + acetyl chloride + AlCl3
Problem 53f
Predict the major products of the following reactions.
(f) p-methylanisole + Br2 , light
Problem 54a
Predict the major products of bromination of the following compounds, using Br2 and FeBr3 in the dark.
(a)
Problem 54b
Predict the major products of bromination of the following compounds, using Br2 and FeBr3 in the dark.
(b)
Problem 54c
Predict the major products of bromination of the following compounds, using Br2 and FeBr3 in the dark.
(c)
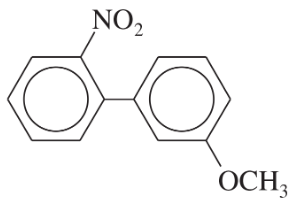
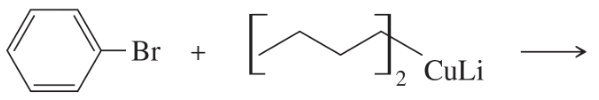
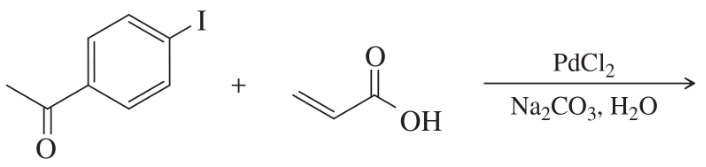
Problem 55a
What products would you expect from the following coupling reactions?
(a)
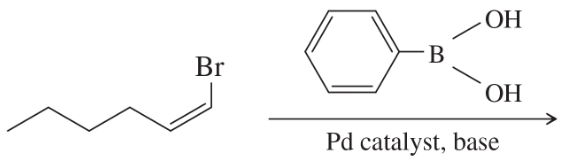
Problem 55b
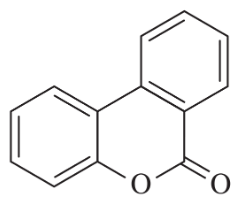
What products would you expect from the following coupling reactions?
(b)
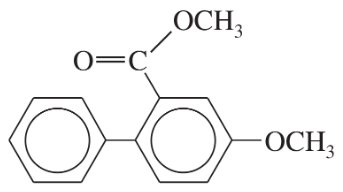
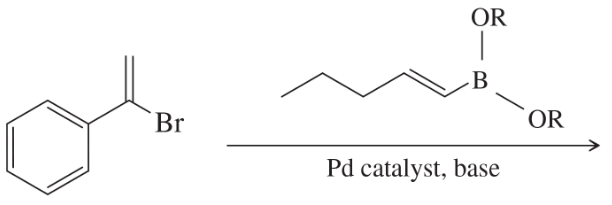
Problem 55c
What products would you expect from the following coupling reactions?
(c)
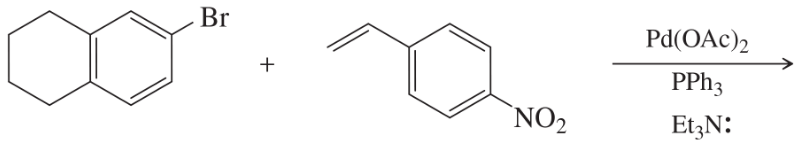
Problem 55d,e
What products would you expect from the following coupling reactions?
(d)
(e)
Problem 56
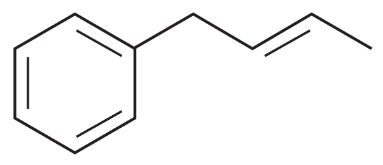
A student added 3-phenylpropanoic acid (PhCH2CH2COOH) to a molten salt consisting of a 1:1 mixture of NaCl and AlCl3 maintained at 170 °C. After 5 minutes, he poured the molten mixture into water and extracted it into dichloromethane. Evaporation of the dichloromethane gave a 96% yield of the product whose spectra follow. The mass spectrum of the product shows a molecular ion at m/z 132. What is the product?
<IMAGE>
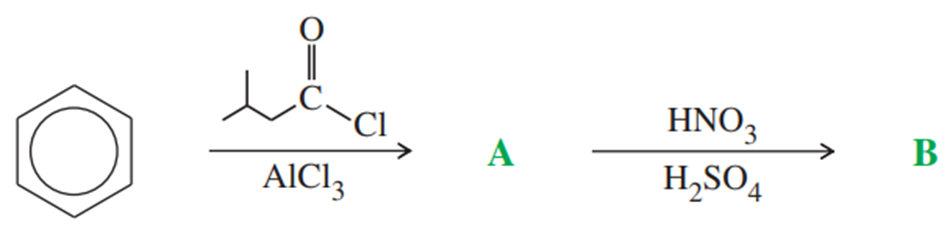
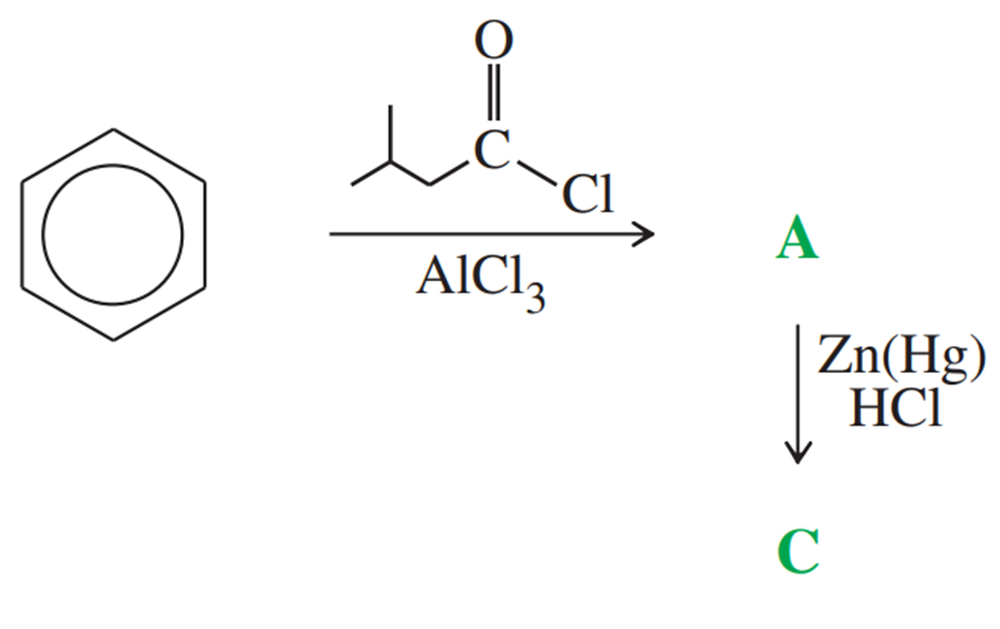
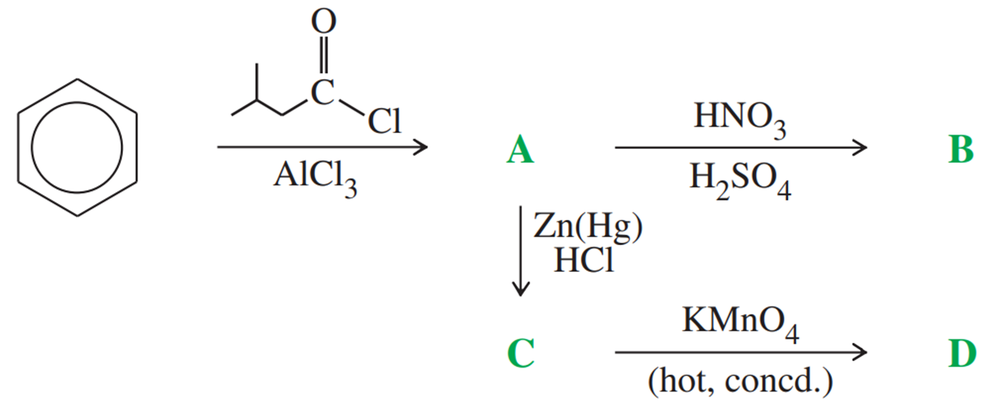
Problem 57a,b
Give the structures of compounds A through B in the following series of reactions.
Problem 57c
Give the structures of compounds A through C in the following series of reactions.
Problem 57d
Give the structures of compounds C through D in the following series of reactions.
Problem 58
The following compound reacts with a hot, concentrated solution of NaOH (in a sealed tube) to give a mixture of two products. Propose structures for these products, and give a mechanism to account for their formation.
Problem 58d
Show the products you expect when each compound reacts with NBS with light shining on the reaction.
(d)
Problem 59
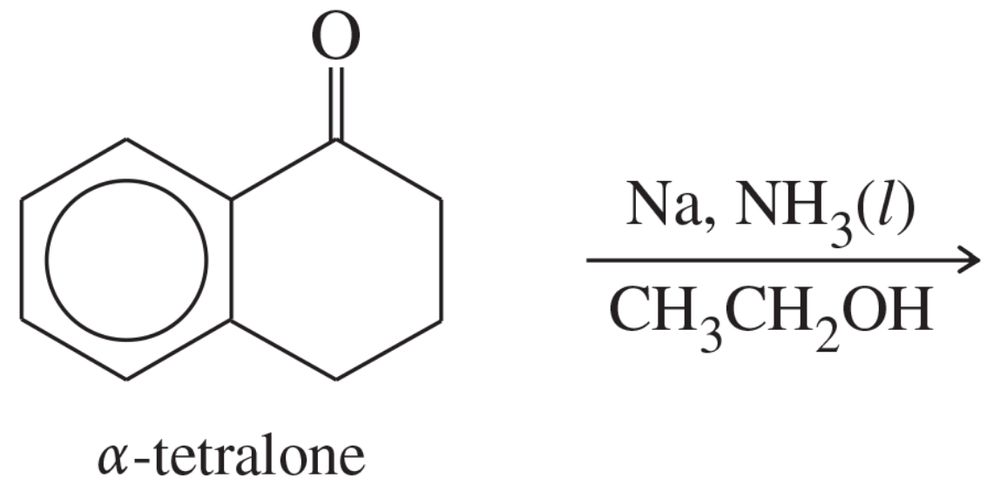
α-Tetralone undergoes Birch reduction to give an excellent yield of a single product. Predict the structure of the product, and propose a mechanism for its formation.
Problem 60a,b,c
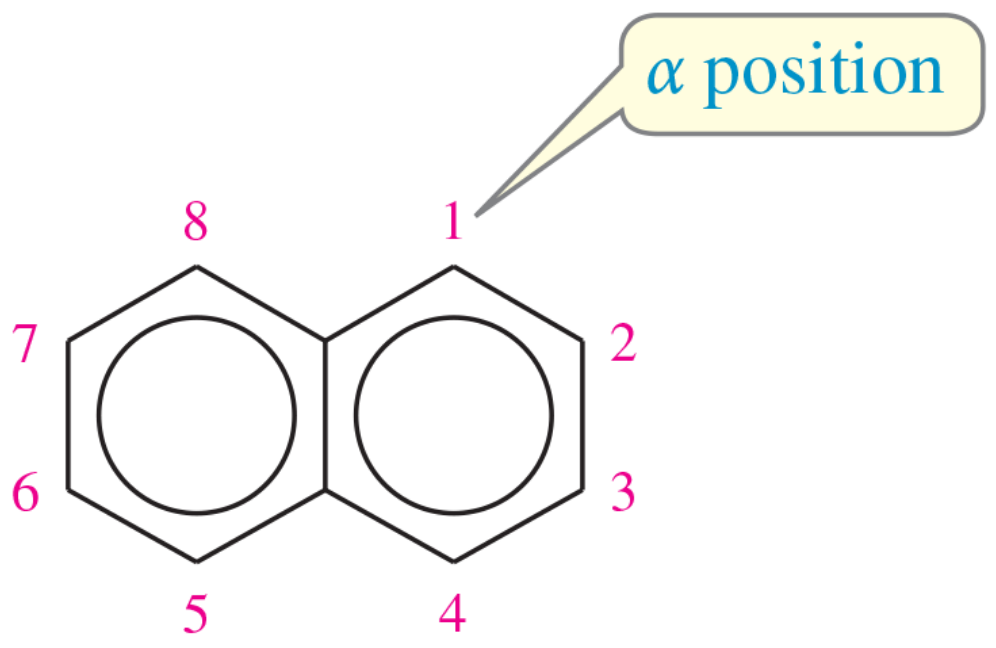
Electrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the a position. Predict the major products of the reactions of naphthalene with the following reagents.
(a) HNO3, H2SO4
(b) Br2, FeBr3
(c) CH3CH2COCl, AlCl3
Problem 60d
Electrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the a position. Predict the major products of the reactions of naphthalene with the following reagents.
(d) isobutylene and HF
Problem 60e
Electrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the 1-position. Predict the major products of the reactions of naphthalene with the following reagents.
(e) cyclohexanol and BF3
Problem 63
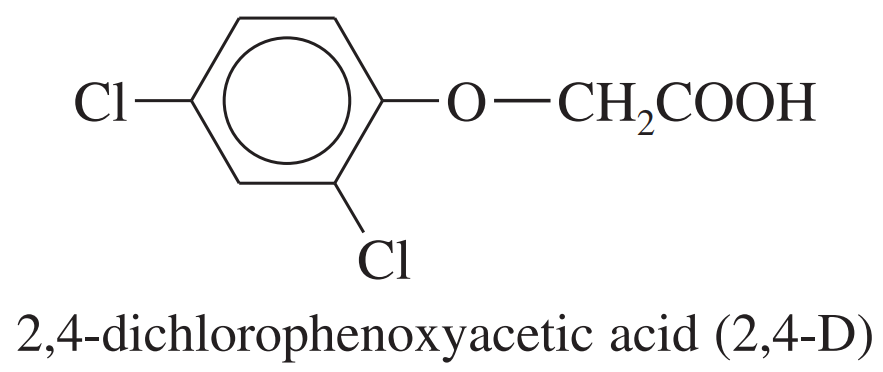
The most common selective herbicide for killing broadleaf weeds is 2,4-dichlorophenoxyacetic acid (2,4-D). Show how you would synthesize 2,4-D from benzene, chloroacetic acid (ClCH2COOH), and any necessary reagents and solvents.
Problem 64a
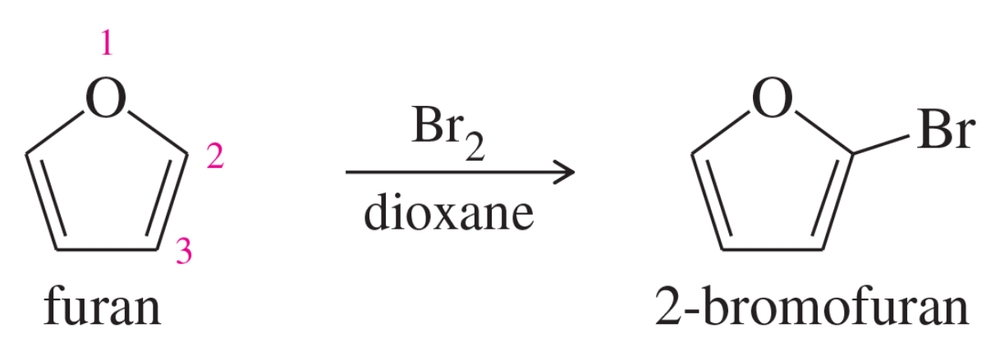
Furan undergoes electrophilic aromatic substitution more readily than benzene; mild reagents and conditions are sufficient. For example, furan reacts with bromine to give 2-bromofuran.
a. Propose mechanisms for the bromination of furan at the 2-position and at the 3-position. Draw the resonance forms of each sigma complex, and compare their stabilities.
Problem 64b
Furan undergoes electrophilic aromatic substitution more readily than benzene; mild reagents and conditions are sufficient. For example, furan reacts with bromine to give 2-bromofuran.
b. Explain why furan undergoes bromination (and other electrophilic aromatic substitutions) primarily at the 2-position.
Problem 65
(a) Draw the three isomers of benzenedicarboxylic acid.
(b) The isomers have melting points of 210 °C, 343 °C, and 427 °C. Nitration of the isomers at all possible positions was once used to determine their structures. The isomer that melts at 210 °C gives two mononitro isomers. The isomer that melts at 343 °C gives three mononitro isomers. The isomer that melts at 427 °C gives only one mononitro isomer. Show which isomer has which melting point.
Problem 67a,b
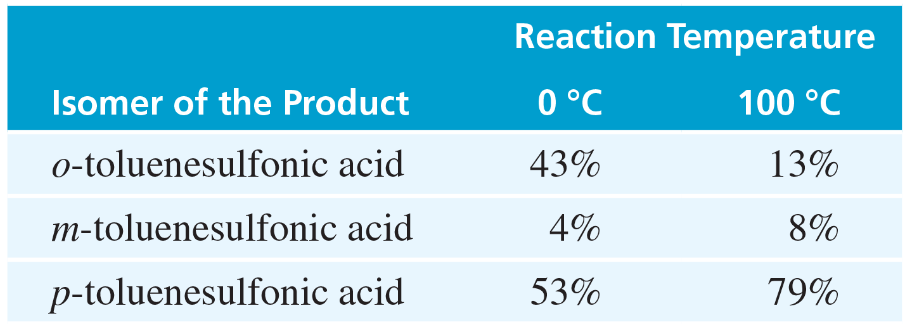
Unlike most other electrophilic aromatic substitutions, sulfonation is often reversible (see Section 17-4). When one sample of toluene is sulfonated at 0 °C and another sample is sulfonated at 100 °C, the following ratios of substitution products result:
a. Explain the change in the product ratios when the temperature is increased.
b. Predict what will happen when the product mixture from the reaction at 0 °C is heated to 100 °C.
Problem 67c
Unlike most other electrophilic aromatic substitutions, sulfonation is often reversible (see Section 17-4). When one sample of toluene is sulfonated at 0 °C and another sample is sulfonated at 100 °C, the following ratios of substitution products result:
c. Because the SO3H group can be added to a benzene ring and removed later, it is sometimes called a blocking group. Show how 2,6-dibromotoluene can be made from toluene using sulfonation and desulfonation as intermediate steps in the synthesis.
Problem 70a
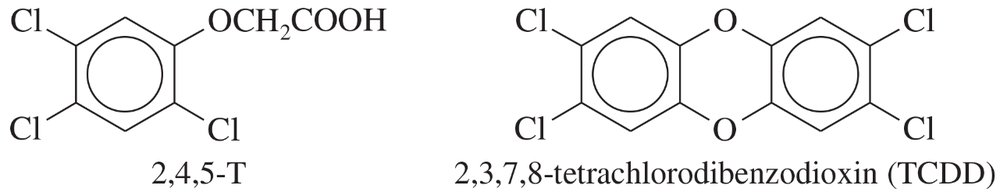
In Chapter 14, we saw that Agent Orange contains (2,4,5-trichlorophenoxy) acetic acid, called 2,4,5-T. This compound is synthesized by the partial reaction of 1,2,4,5-tetrachlorobenzene with sodium hydroxide, followed by reaction with sodium chloroacetate, ClCH2CO2Na.
a. Draw the structures of these compounds, and write equations for these reactions.
Problem 71
Phenol reacts with three equivalents of bromine in CCl4 (in the dark) to give a product of formula C6H3OBr3. When this product is added to bromine water, a yellow solid of molecular formula C6H2OBr4 precipitates out of the solution. The IR spectrum of the yellow precipitate shows a strong absorption (much like that of a quinone) around 1680 cm–1. Propose structures for the two products.