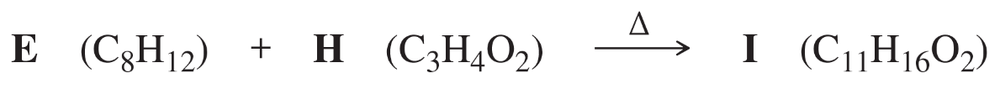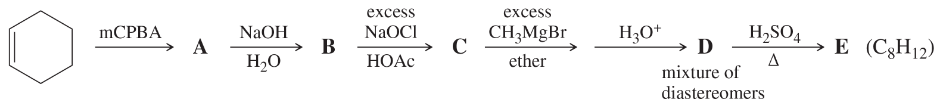Back
Back Wade 9th Edition
Wade 9th Edition Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet SpectroscopyProblem 33c,d
Show how Diels–Alder reactions might be used to synthesize the following compounds.
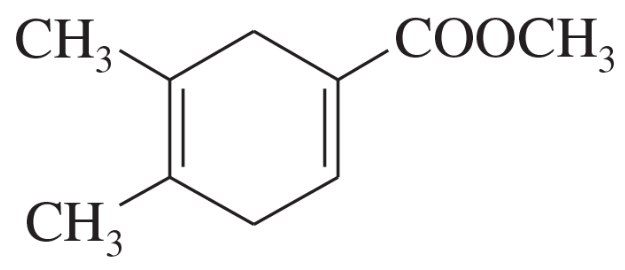
(c)
(d)
Problem 33g,h
Show how Diels–Alder reactions might be used to synthesize the following compounds.
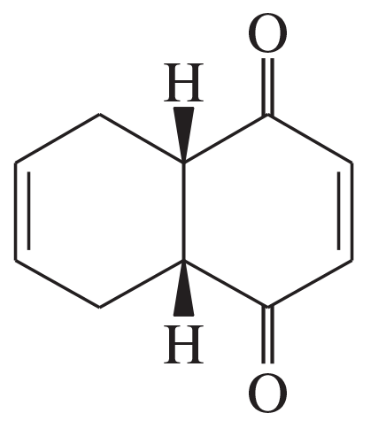
(g)
(h)
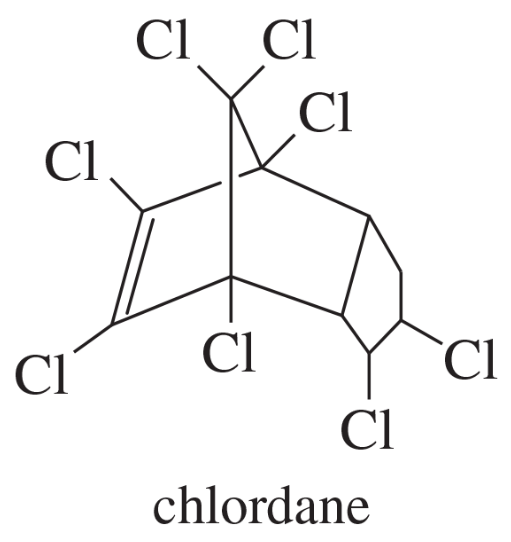
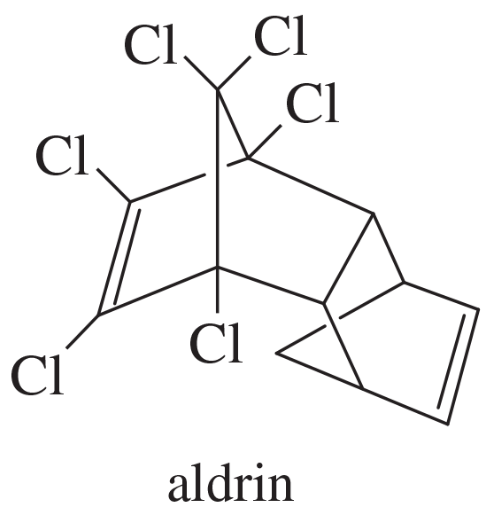
Problem 33i
Show how Diels–Alder reactions might be used to synthesize the following compounds.
(i)
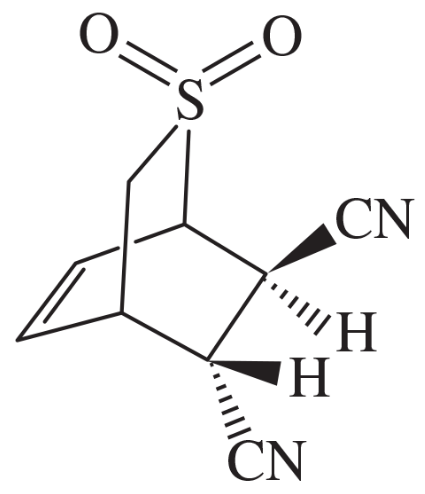
Problem 34(3)
Give the structures of the products represented by letters in this synthesis.
Part 3:
Problem 34a
Give the structures of the products represented by letters in this synthesis.
Part 1:
Problem 34b
Give the structures of the products represented by letters in this synthesis.
Part 2:
Problem 35a
Furan and maleimide undergo a Diels–Alder reaction at 25 °C to give the endo isomer of the product. When the reaction takes place at 90 °C, however, the major product is the exo isomer. Further study shows that the endo isomer of the product isomerizes to the exo isomer at 90 °C.
a. Draw and label the endo and exo isomers of the Diels–Alder adduct of furan and maleimide.
Problem 35b
Furan and maleimide undergo a Diels–Alder reaction at 25 °C to give the endo isomer of the product. When the reaction takes place at 90 °C, however, the major product is the exo isomer. Further study shows that the endo isomer of the product isomerizes to the exo isomer at 90 °C.
b. Which isomer of the product would you usually expect from this reaction? Explain why this isomer is usually favored.
Problem 35c
Furan and maleimide undergo a Diels–Alder reaction at 25 °C to give the endo isomer of the product. When the reaction takes place at 90 °C, however, the major product is the exo isomer. Further study shows that the endo isomer of the product isomerizes to the exo isomer at 90 °C.
c. Examine your answer to (b) and determine whether this answer applies to a reaction that is kinetically controlled or one that is thermodynamically controlled, or both.
Problem 36a
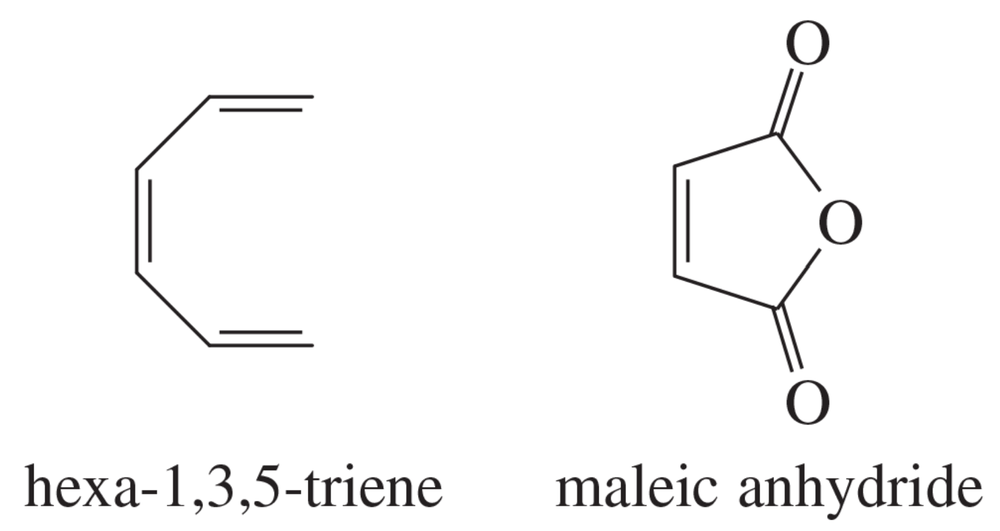
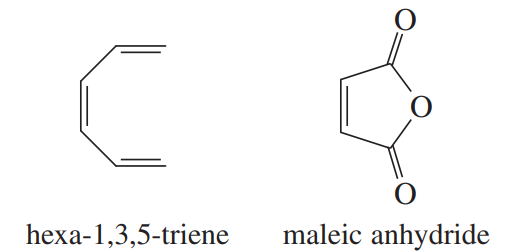
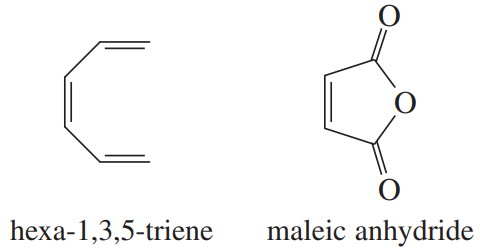
Sketch the pi molecular orbitals of hexa-1,3,5-triene.
Problem 36b
Show the electronic configuration of the ground state of hexa-1,3,5-triene.
Problem 36c
Show what product would result from the [6 + 2] cycloaddition of hexa-1,3,5-triene with maleic anhydride.
Problem 36d
Show that the [6 + 2] cyclization of hexa-1,3,5-triene with maleic anhydride is thermally forbidden but photochemically allowed.
Problem 36e
Show the Diels–Alder product that would actually result from heating hexa-1,3,5-triene with maleic anhydride.
Problem 37a
The pentadienyl radical, H2C=CH–CH=CH–CH2•, has its unpaired electron delocalized over three carbon atoms.
a. Use resonance forms to show which three carbon atoms bear the unpaired electron.
Problem 37b,c,d
The pentadienyl radical, H2C=CH–CH=CH–CH2•, has its unpaired electron delocalized over three carbon atoms.
b. How many MOs are there in the molecular orbital picture of the pentadienyl radical?
c. How many nodes are there in the lowest-energy MO of the pentadienyl system? How many in the highest-energy MO?
d. Draw the MOs of the pentadienyl system in order of increasing energy
Problem 37f
The pentadienyl radical, H2C=CH–CH=CH–CH2•, has its unpaired electron delocalized over three carbon atoms.
f. Show how your molecular orbital picture agrees with the resonance picture showing delocalization of the unpaired electron onto three carbon atoms.
Problem 37g
The pentadienyl radical, H2C=CH–CH=CH–CH2•, has its unpaired electron delocalized over three carbon atoms.
g. Remove the highest-energy electron from the pentadienyl radical to give the pentadienyl cation. Which carbon atoms share the positive charge? Does this picture agree with the resonance picture?
Problem 37h
The pentadienyl radical, H2C=CH–CH=CH–CH2•, has its unpaired electron delocalized over three carbon atoms.
h. Add an electron to the pentadienyl radical to give the pentadienyl anion. Which carbon atoms share the negative charge? Does this picture agree with the resonance picture?
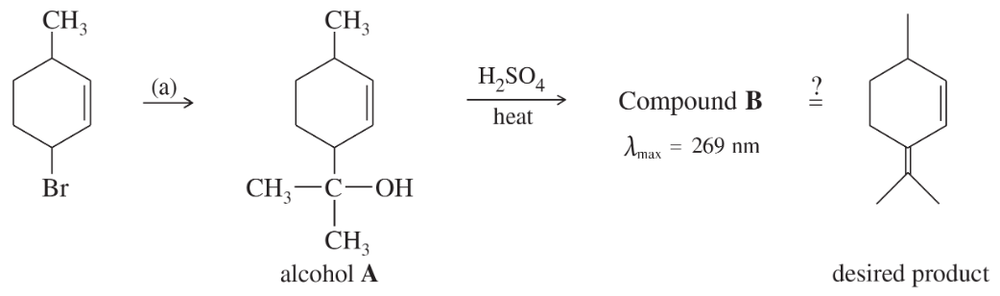
Problem 38a
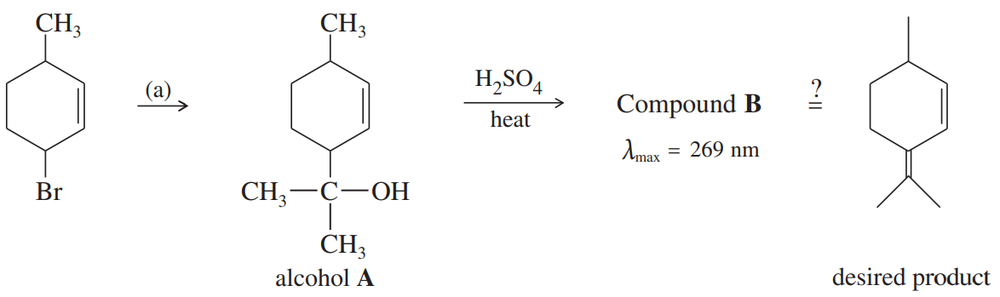
A student was studying terpene synthesis, and she wanted to make the compound shown here. First she converted 3-bromo-6-methylcyclohexene to alcohol A. She heated alcohol A with sulfuric acid and purified one of the components (compound B) from the resulting mixture. Compound B has the correct molecular formula for the desired product.
a. Suggest how 3-bromo-6-methylcyclohexene might be converted to alcohol A.
Problem 38c
A student was studying terpene synthesis, and she wanted to make the compound shown here. First she converted 3-bromo-6-methylcyclohexene to alcohol A. She heated alcohol A with sulfuric acid and purified one of the components (compound B) from the resulting mixture. Compound B has the correct molecular formula for the desired product.
(c) Propose a mechanism for the dehydration of alcohol A to compound B.
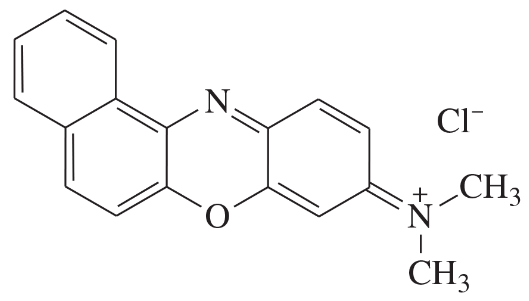
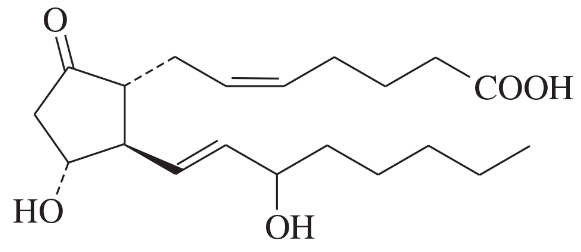
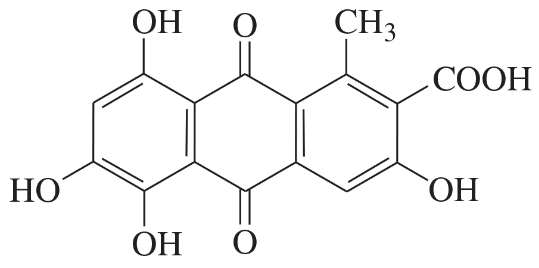
Problem 40a,b,c
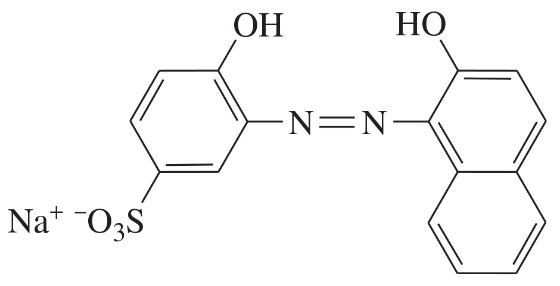
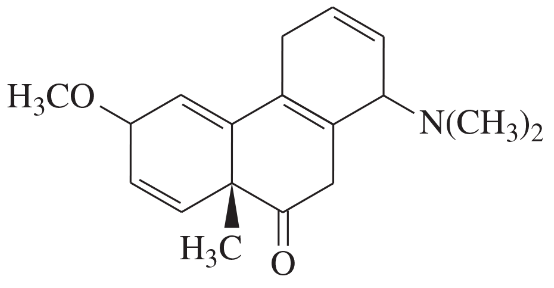
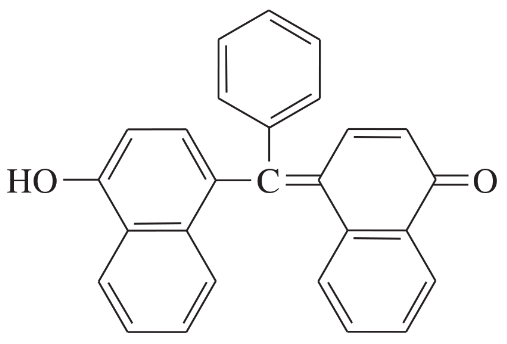
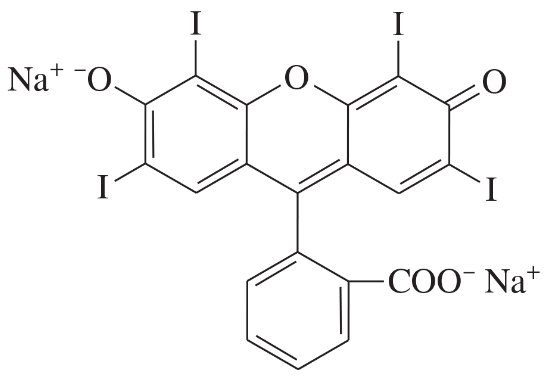
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(a)
(b)
(c)
Problem 40d,e,f
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(d)
(e)
(f)
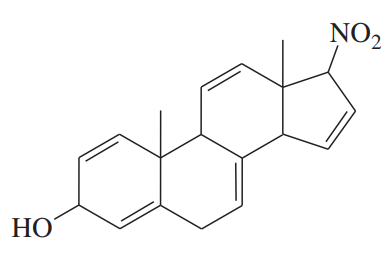
Problem 40g
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(g)
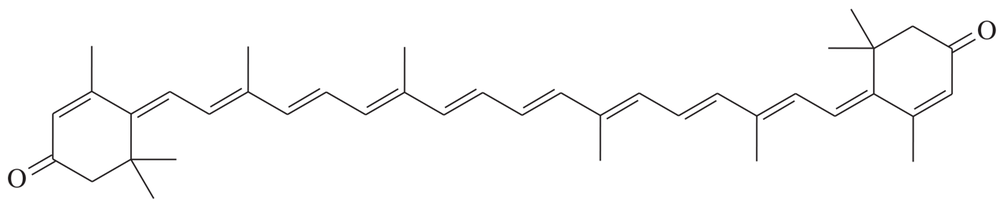
Problem 40h
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(h)
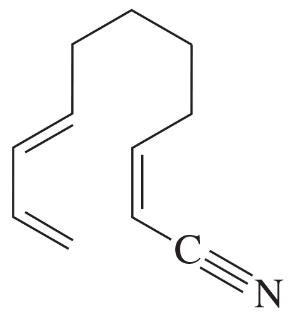
Problem 40i
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(i)
Problem 41a,b
An important variation of the Diels–Alder reaction is intramolecular, in which the diene and the dienophile are connected. This type of Diels–Alder reaction makes two new rings. Draw the compound produced in each of these examples; try to predict stereochemistry (using models will help). In some cases, Lewis acid catalysts are used; that can be ignored for this problem.
(a)
(b)
Problem 41c
An important variation of the Diels–Alder reaction is intramolecular, in which the diene and the dienophile are connected. This type of Diels–Alder reaction makes two new rings. Draw the compound produced in each of these examples; try to predict stereochemistry (using models will help). In some cases, Lewis acid catalysts are used; that can be ignored for this problem.
(c)
Problem 41d
An important variation of the Diels–Alder reaction is intramolecular, in which the diene and the dienophile are connected. This type of Diels–Alder reaction makes two new rings. Draw the compound produced in each of these examples; try to predict stereochemistry (using models will help). In some cases, Lewis acid catalysts are used; that can be ignored for this problem.
(d)