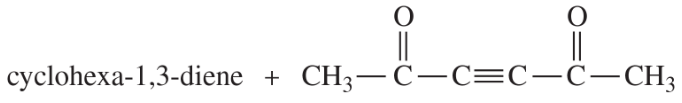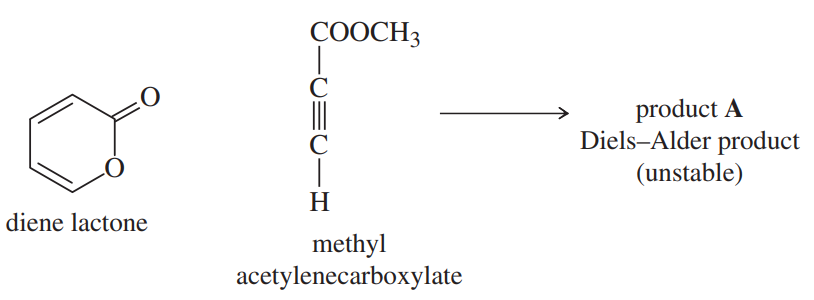Back
Back Wade 9th Edition
Wade 9th Edition Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet SpectroscopyProblem 20a
Show that the [4 + 4] cycloaddition of two butadiene molecules to give cycloocta-1,5-diene is thermally forbidden but photochemically allowed.
Problem 20b
There is a different, thermally allowed cycloaddition of two butadiene molecules. Show this reaction, and explain why it is thermally allowed. (Hint: Consider the dimerization of cyclopentadiene.)
Problem 21
One milligram of a compound of molecular weight 160 is dissolved in 10 mL of ethanol, and the solution is poured into a 1-cm UV cell. The UV spectrum is taken, and there is an absorption at λmax = 247 nm. The maximum absorbance at 247 nm is 0.50. Calculate the value of e for this absorption.
Problem 22
Match four of the following UV absorption maxima (λmax) with the corresponding compounds: (1) 232 nm; (2) 256 nm; (3) 273 nm; (4) 292 nm; (5) 313 nm; (6) 353 nm.
Problem 23
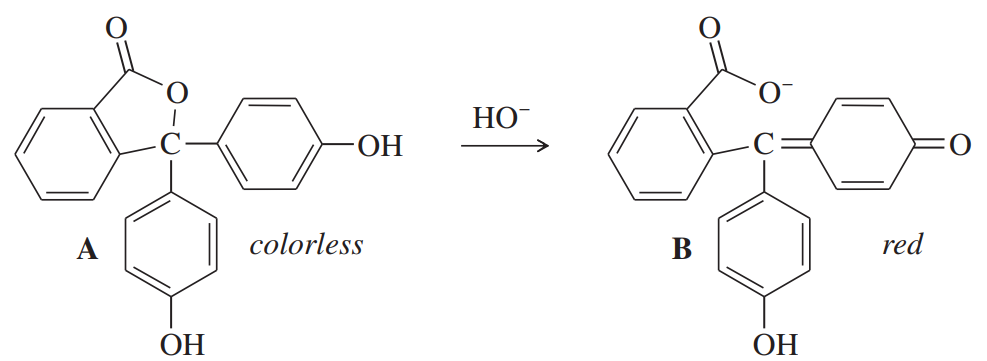
Phenolphthalein is an acid–base indicator that is colorless below pH 8 and red above pH 8. Explain briefly why the first structure is colorless and the second structure is colored.
Problem 24
Classify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of these classifications.
(a) cycloocta-1,4-diene
(b) cycloocta-1,3-diene
(c) cyclodeca-1,2-diene
(d) cycloocta-1,3,5,7-tetraene
(e) cyclohexa-1,3,5-triene (benzene)
(f) penta-1,2,4-triene
Problem 25a
Predict the products of the following reactions.
(a) allyl bromide + cyclohexyl magnesium bromide
Problem 25b
Predict the products of the following reactions.
(b) cyclopentadiene + anhydrous HCl
Problem 25c
Predict the products of the following reactions.
(c) 2-methylpropene + NBS, light
Problem 25d
Predict the products of the following reactions.
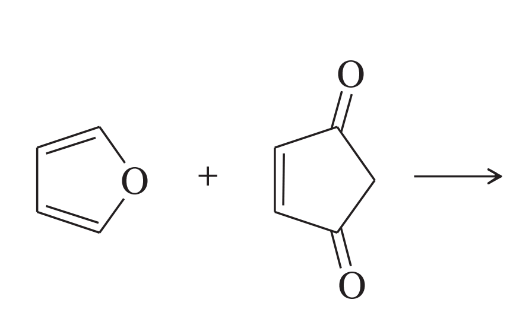
(d) furan + trans-1,2-dicyanoethylene
Problem 25e
Predict the products of the following reactions.
(e) buta-1,3-diene + bromine water
Problem 25f
Predict the products of the following reactions.
(f) hexa-1,3,5-triene + bromine in CCl4
Problem 25g
Predict the products of the following reactions.
(g) 1-(bromomethyl)-2-methylcyclopentene, heated in methanol
Problem 25h
Predict the products of the following reactions.
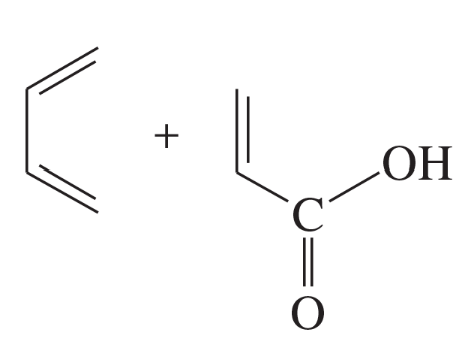
(h) cyclopentadiene + methyl acrylate, CH2=CH–COOCH3
Problem 25i
Predict the products of the following reactions.
(i)
Problem 26a
Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following hydrocarbons.
a. 5-methylhex-1-ene
Problem 26b
Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following hydrocarbons.
b. 2,5,5-trimethylhept-2-ene
Problem 26c
Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following hydrocarbons.
c. 1-cyclopentylpent-2-ene
Problem 27a
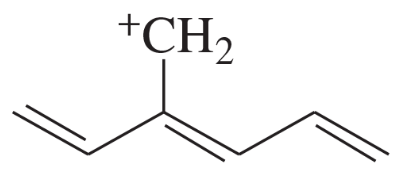
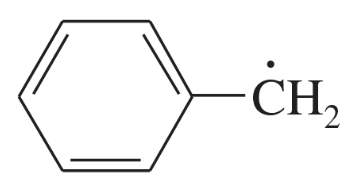
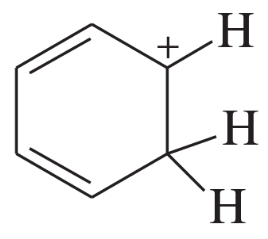
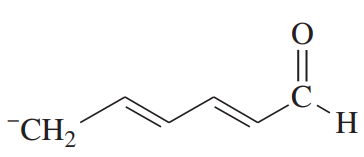
Draw the important resonance contributors for the following cations, anions, and radicals.
(a)
Problem 27b
Draw the important resonance contributors for the following cations, anions, and radicals.
(b)
Problem 27c
Draw the important resonance contributors for the following cations, anions, and radicals.
(c)
Problem 27d
Draw the important resonance contributors for the following cations, anions, and radicals.
(d)
Problem 27e,f
Draw the important resonance contributors for the following cations, anions, and radicals.
(e)
(f)
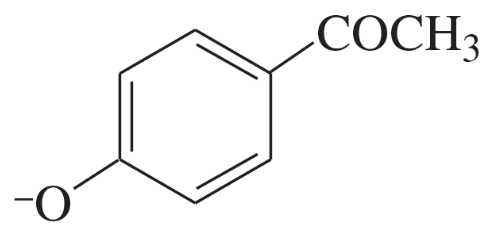
Problem 28
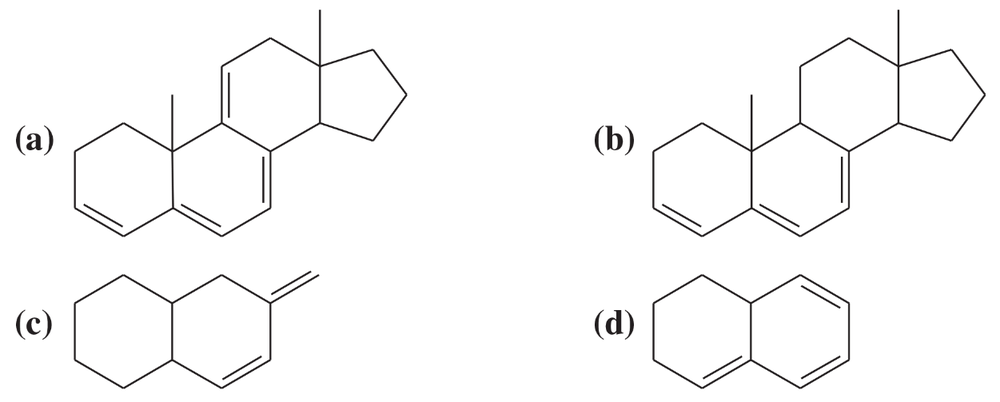
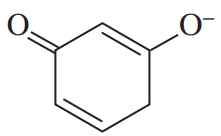
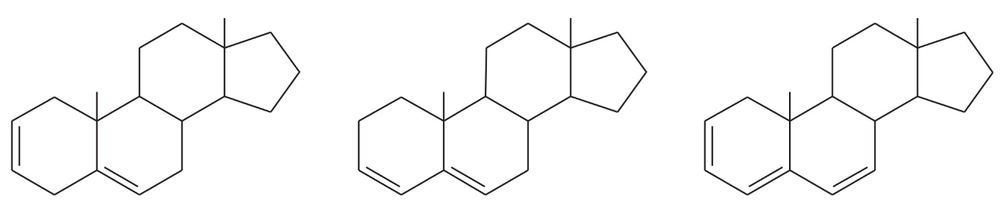
A solution was prepared using 0.0010 g of an unknown steroid (of molecular weight around 255) in 100 mL of ethanol. Some of this solution was placed in a 1-cm cell, and the UV spectrum was measured. This solution was found to have λmax = 235 nm, with A = 0.74.
(a) Compute the value of the molar absorptivity at 235 nm.
(b) Which of the following compounds might give this spectrum?
Problem 29b
The diene lactone shown in part (a) has one electron-donating group (-OR) and one electron-withdrawing group (C=O). This diene lactone is sufficiently electron-rich to serve as the diene in a Diels–Alder reaction.
b. The Diels–Alder product A is not very stable. Upon mild heating, it reacts to produce CO2 gas and methyl benzoate (PhCOOCH3), a very stable product. Explain how this strongly exothermic decarboxylation takes place. (Hint: Under the right conditions, the Diels–Alder reaction can be reversible.)
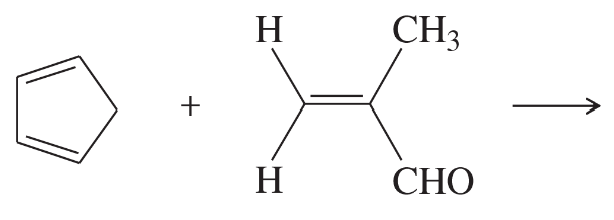
Problem 30a
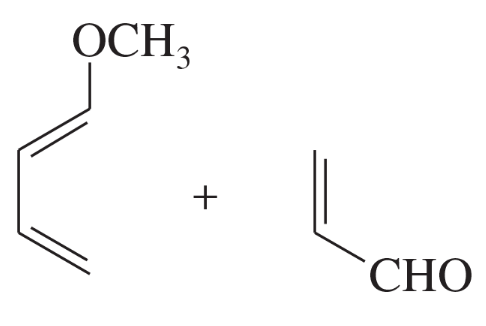
Predict the products of the following proposed Diels–Alder reactions. Include stereochemistry where appropriate.
(a)
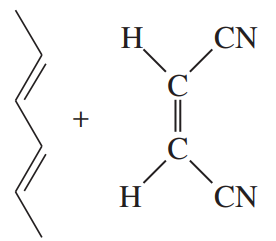
Problem 30d
Predict the products of the following proposed Diels–Alder reactions. Include stereochemistry where appropriate.
(d)
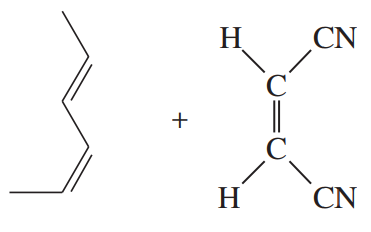
Problem 30e,f
Predict the products of the following proposed Diels–Alder reactions. Include stereochemistry where appropriate.
(e)
(f)
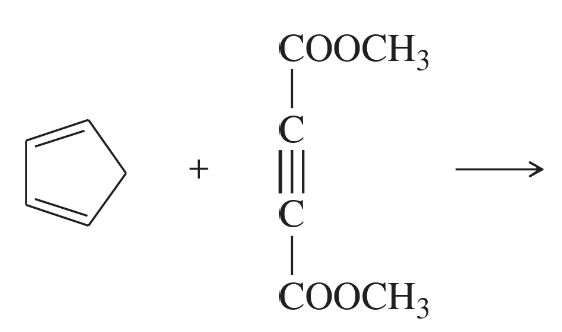
Problem 31a,b,c
Predict the products of the following proposed Diels–Alder reactions. Include stereochemistry where appropriate.
(a)
(b)
(c)
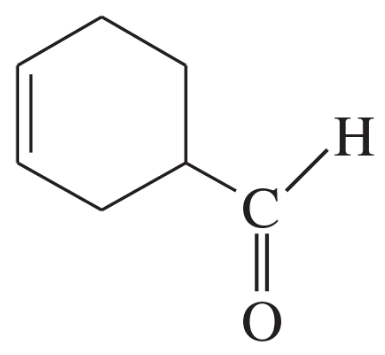
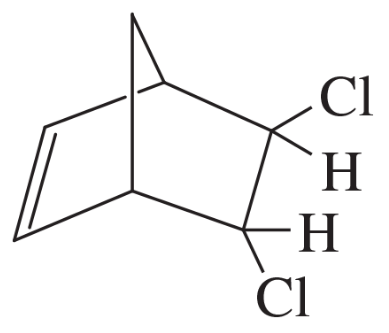
Problem 33a,b
Show how Diels–Alder reactions might be used to synthesize the following compounds.
(a)
(b)