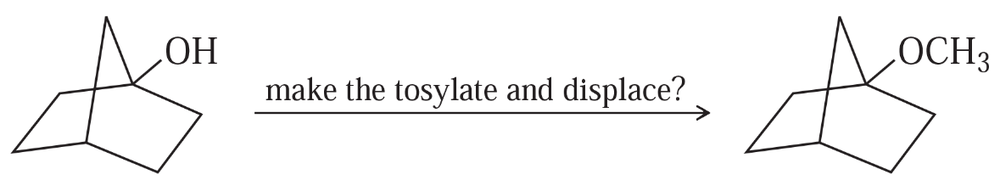Back
BackProblem 56a,b,c
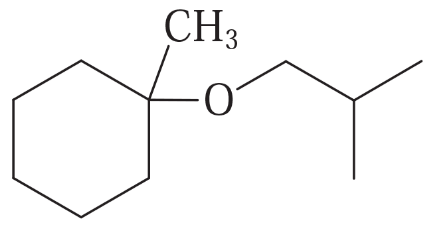
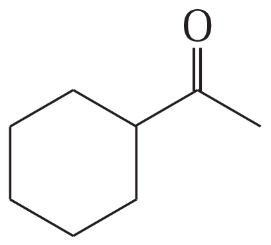
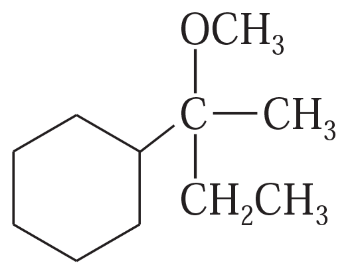
Show how you would synthesize the following compounds. As starting materials, you may use any alcohols containing four or fewer carbon atoms, cyclohexanol, and any necessary solvents and inorganic reagents.
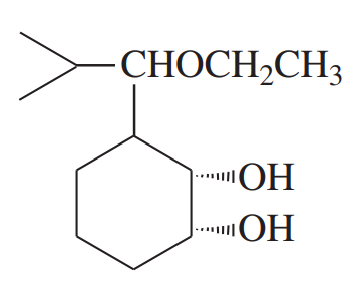
(a)
(b)
(c)
Problem 56g
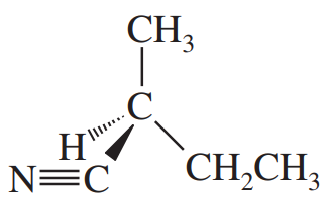
Show how you would synthesize the following compounds. As starting materials, you may use any alcohols containing four or fewer carbon atoms, cyclohexanol, and any necessary solvents and inorganic reagents.
(g)
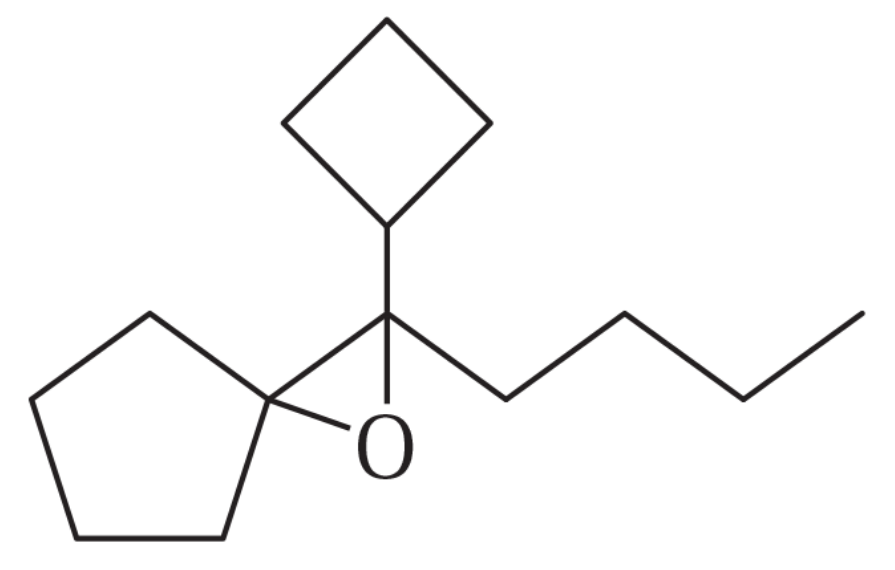
Problem 56h
Show how you would synthesize the following compounds. As starting materials, you may use any alcohols containing four or fewer carbon atoms, cyclohexanol, and any necessary solvents and inorganic reagents.
(h)
Problem 57
Show how you would synthesize the following compound. As starting materials, you may use any alcohols containing five or fewer carbon atoms and any necessary solvents and inorganic reagents.
Problem 58
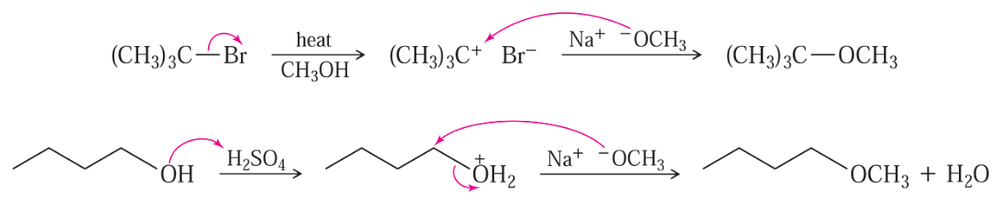
The following pseudo-syntheses (guaranteed not to work) exemplify a common conceptual error.
(a) What is the conceptual error implicit in these syntheses?
(b) Propose syntheses that are more likely to succeed.
Problem 59
Two unknowns, X and Y, both having the molecular formula C4H8O, give the following results with four chemical tests. Propose structures for X and Y consistent with this information.
Problem 60
The Williamson ether synthesis involves the displacement of an alkyl halide or tosylate by an alkoxide ion. Would the synthesis shown be possible by making a tosylate and displacing it? If so, show the sequence of reactions. If not, explain why not and show an alternative synthesis that would be more likely to work.
Problem 61
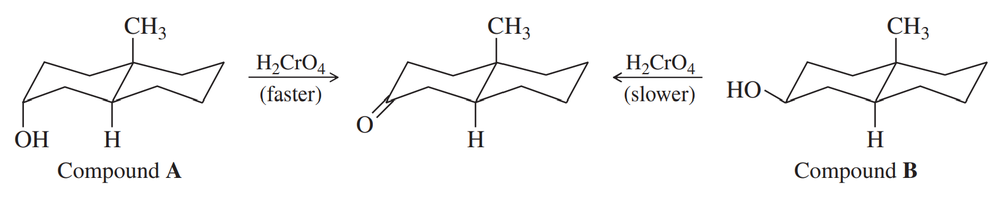
Chromic acid oxidation of an alcohol (Section 11-2A) occurs in two steps: formation of the chromate ester, followed by an elimination of H+ and chromium. Which step do you expect to be rate-limiting? Careful kinetic studies have shown that Compound A undergoes chromic acid oxidation over 10 times as fast as Compound B. Explain this large difference in rates.
Problem 62a
(a) The reaction of butan-2-ol with concentrated aqueous HBr goes with partial racemization, giving more inversion than retention of configuration. Propose a mechanism that accounts for racemization with excess inversion.
Problem 62b
(b) Under the same conditions, an optically active sample of trans-2-bromocyclopentanol reacts with concentrated aqueous HBr to give an optically inactive product, (racemic) trans-1,2-dibromocyclopentane. Propose a mechanism to show how this reaction goes with apparently complete retention of configuration, yet with racemization. (Hint: Draw out the mechanism of the reaction of cyclopentene with Br2 in water to give the starting material, trans-2- bromocyclopentanol. Consider how parts of this mechanism might be involved in the reaction with HBr.)
Problem 63a
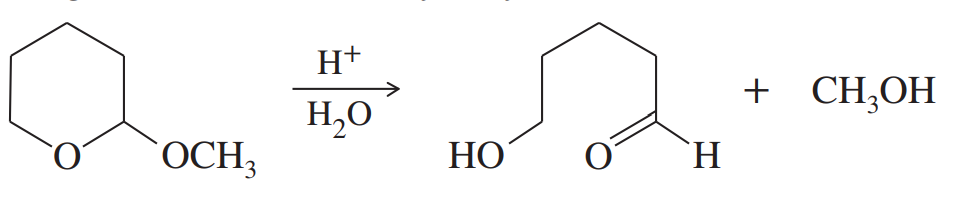
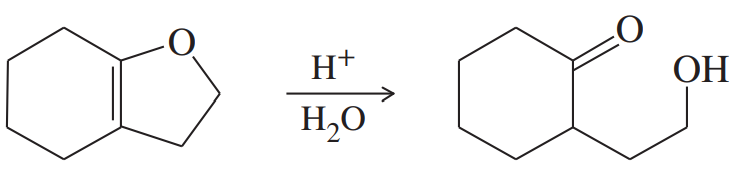
Alcohols combine with ketones and aldehydes to form interesting derivatives, which we will discuss in Chapter 18. The following reactions show the hydrolysis of two such derivatives. Propose mechanisms for these reactions.
(a)
Problem 63b
Alcohols combine with ketones and aldehydes to form interesting derivatives, which we will discuss in Chapter 18. The following reactions show the hydrolysis of two such derivatives. Propose mechanisms for these reactions.
(b)
Problem 66a,b
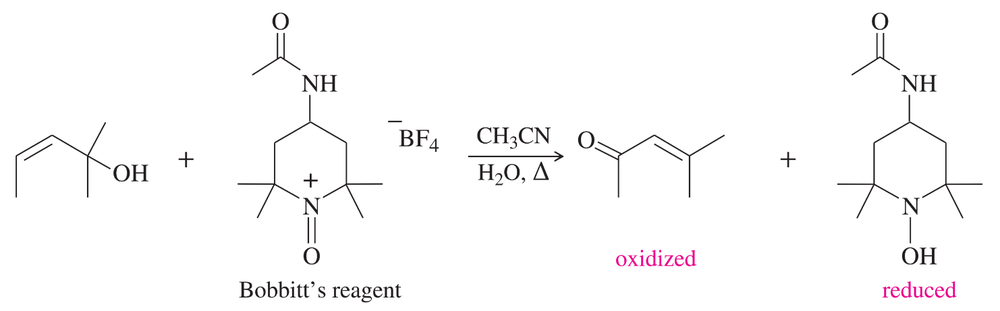
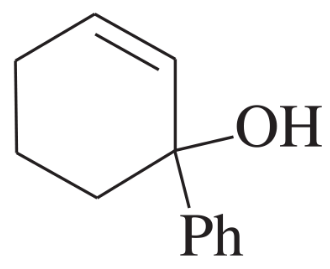
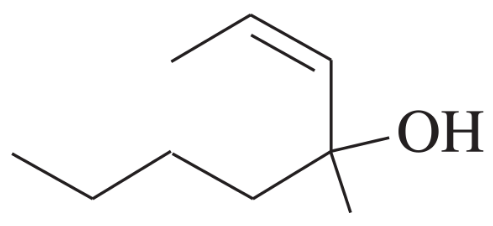
Under normal circumstances, tertiary alcohols are not oxidized. However, when the tertiary alcohol is allylic, it can undergo a migration of the double bond (called an allylic shift) and subsequent oxidation of the alcohol. A particularly effective reagent for this reaction is Bobbitt's reagent, similar to TEMPO used in many oxidations. (M. Shibuya et al., J. Org. Chem., 2008, 73, 4750.)
Show the expected product when each of these 3° allylic alcohols is oxidized by Bobbitt's reagent.
(a)
(b)
Problem 66c,d
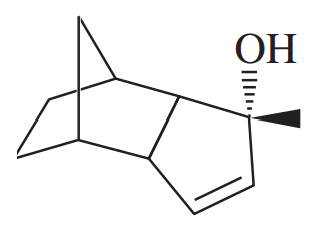
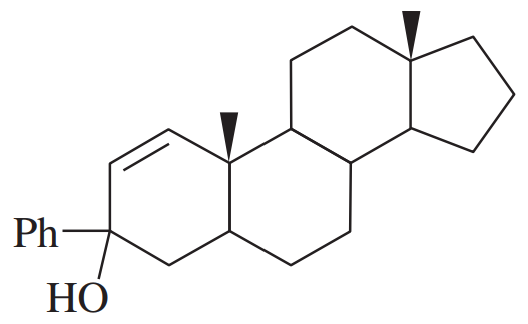
Under normal circumstances, tertiary alcohols are not oxidized. However, when the tertiary alcohol is allylic, it can undergo a migration of the double bond (called an allylic shift) and subsequent oxidation of the alcohol. A particularly effective reagent for this reaction is Bobbitt's reagent, similar to TEMPO used in many oxidations. (M. Shibuya et al., J. Org. Chem., 2008, 73, 4750.)
Show the expected product when each of these 3° allylic alcohols is oxidized by Bobbitt’s reagent
(c)
(d)