Back
BackProblem 2
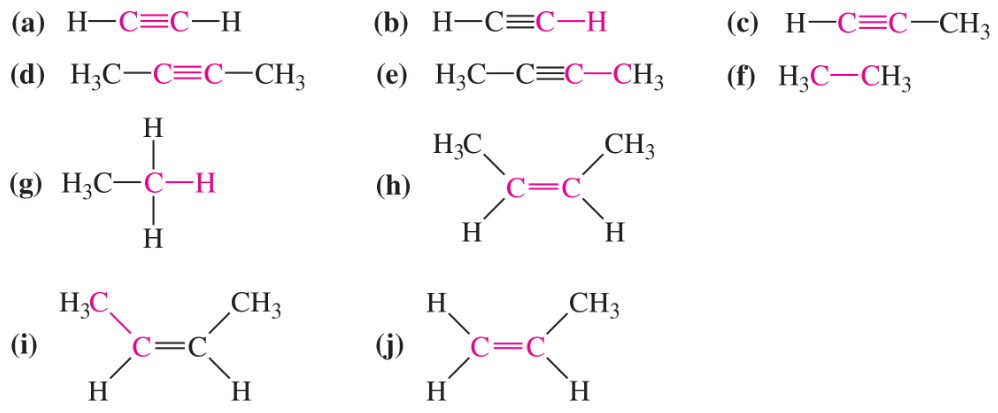
Which of the bonds shown in red are expected to have IR-active stretching frequencies?
Problem 4
Spectra are given for three compounds. Each compound has one or more of the following functional groups: alcohol, amine, ketone, aldehyde, and carboxylic acid. Determine the functional group(s) in each compound, and assign the major peaks above 1600 cm–1.
<IMAGE>
Problem 5
The infrared spectra for three compounds are provided. Each compound has one or more of the following functional groups: conjugated ketone, ester, amide, nitrile, and alkyne. Determine the functional group(s) in each compound, and assign the major peaks above 1600 cm–1.
<IMAGE>
Problem 7
Identify which of these four mass spectra indicate the presence of sulfur, chlorine, bromine, iodine, or nitrogen. Suggest a molecular formula for each.
<IMAGE>
Problem 9
Show the fragmentations that give rise to the peaks at m/z 43, 57, and 85 in the mass spectrum of 2,4-dimethylpentane (Figure 12-17).
<IMAGE>
Problem 10
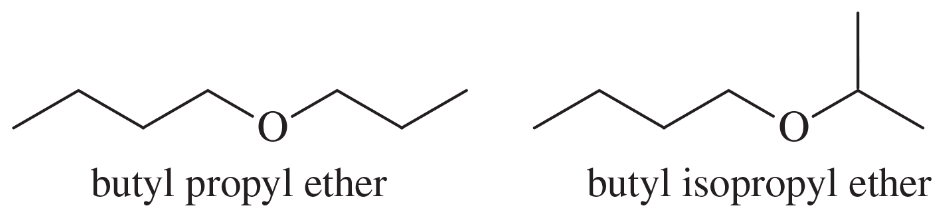
Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar but distinctive mass spectra.
Both compounds give prominent peaks at m/z 116, 73, 57, and 43. But one compound gives a distinctive strong peak at 87, and the other compound gives a strong peak at 101. Determine which compound gives the peak at 87 and which one gives the peak at 101. Propose fragmentations to account for the ions at m/z 116, 101, 87, and 73.
Problem 11
Account for the peaks at m/z 87, 111, and 126 in the mass spectrum of 2,6-dimethylheptan-4-ol.
<IMAGE>
Problem 12a,b,c
Predict the characteristic infrared absorptions of the functional groups in the following molecules.
(a) cyclohexene
(b) pentan-2-ol
(c) pentan-2-one
Problem 12d,e,f
Predict the characteristic infrared absorptions of the functional groups in the following molecules.
(d) pent-1-yne
(e) diethylamine
(f) pentanoic acid
Problem 13a,b,c
Convert the following infrared wavelengths to cm-1. (a) 6.24 𝜇m, typical for an aromatic C=C (b) 3.38 𝜇m, typical for a saturated C-H bond (c) 5.85 𝜇m, typical for a ketone carbonyl
Problem 16a
Four infrared spectra are shown, corresponding to four of the following compounds. For each spectrum, determine the structure and explain how the peaks in the spectrum correspond to the structure you have chosen.
<IMAGE>
Problem 17a
Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the following compounds. (a) 2-methylpentane
Problem 17b
Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the following compounds.
(b) 3-methylhex-2-ene
Problem 17c
Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the following compounds.
(c) 4-methylpentan-2-ol
Problem 19a,b
A common lab experiment is the dehydration of cyclohexanol to cyclohexene.
(a) Explain how you could tell from the IR spectrum whether your product was pure cyclohexene, pure cyclohexanol, or a mixture of cyclohexene and cyclohexanol. Give approximate frequencies for distinctive peaks.
(b) Explain why mass spectrometry might not be a good way to distinguish cyclohexene from cyclohexanol.
Problem 21a,b
A C-D (carbon–deuterium) bond is electronically much like a C-H bond, and it has a similar stiffness, measured by the spring constant, k. The deuterium atom has twice the mass (m) of a hydrogen atom, however.
(a) The infrared absorption frequency is approximately proportional to , when one of the bonded atoms is much heavier than the other, and m is the lighter of the two atoms (H or D in this case). Use this relationship to calculate the IR absorption frequency of a typical C-D bond. Use 3000 cm–1 as a typical C-H absorption frequency.
(b) A chemist dissolves a sample in deuterochloroform (CDCl3) and then decides to take the IR spectrum and simply evaporates most of the CDCl3. What functional group will appear to be present in this IR spectrum as a result of the CDCl3 impurity?
Problem 26a
A laboratory student added 1-bromobutane to a flask containing dry ether and magnesium turnings. An exothermic reaction resulted, and the ether boiled vigorously for several minutes. Then she added acetone to the reaction mixture and the ether boiled even more vigorously. She added dilute acid to the mixture and separated the layers. She evaporated the ether layer, and distilled a liquid that boiled at 143 °C. GC–MS analysis of the distillate showed one major product with a few minor impurities. The mass spectrum of the major product is shown here.
(a) Draw out the reactions that took place and show the product that was formed.
<IMAGE>
Problem 26b
A laboratory student added 1-bromobutane to a flask containing dry ether and magnesium turnings. An exothermic reaction resulted, and the ether boiled vigorously for several minutes. Then she added acetone to the reaction mixture and the ether boiled even more vigorously. She added dilute acid to the mixture and separated the layers. She evaporated the ether layer, and distilled a liquid that boiled at 143 °C. GC–MS analysis of the distillate showed one major product with a few minor impurities. The mass spectrum of the major product is shown here.
(b) Explain why the molecular ion is or is not visible in the mass spectrum, and show what ions are likely to be responsible for the strong peaks at m/z 59 and 101.
<IMAGE>
Problem 28
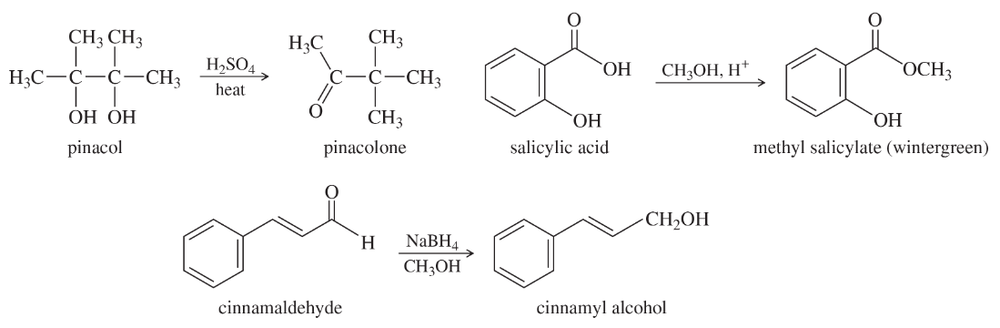
Three common lab experiments are shown. In each case, describe how the IR spectrum of the product would differ from that of the reactant. Give approximate frequencies for distinctive peaks in the IR spectrum of the reactant and also that of the product.
Problem 29
The ultimate test of fluency in MS and IR is whether you can determine a moderately complex structure from just the MS and the IR, with no additional information. The IR and MS of a compound are shown below. Use everything you know about IR and MS, plus reasoning and intuition, to determine a likely structure. Then show how your proposed structure is consistent with these spectra.
<IMAGES>
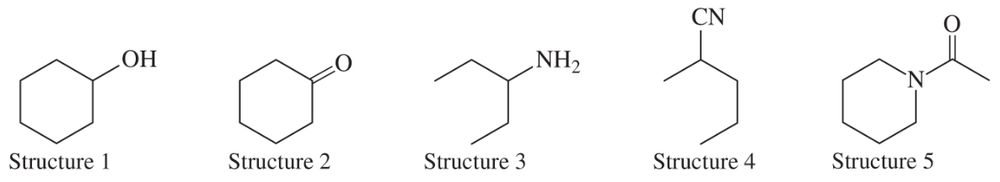
Problem 30
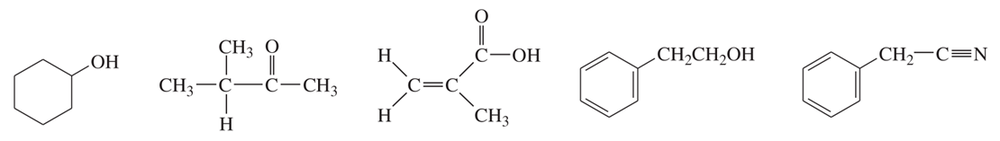
These five structures all have distinguishing absorptions in the IR. Match each structure with its characteristic absorption.
(a) sharp, 2254 cm–1
(b) very broad, centered about 3330 cm–1
(c) strong, slightly broadened, 1645 cm–1
(d) broad with spikes at 3367 and 3292 cm–1
(e) strong, sharp 1717 cm–1
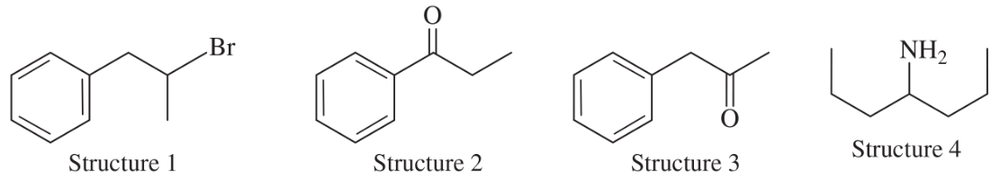
Problem 31
Consider the following four structures, followed by mass spectral data. Match each structure with its characteristic molecular ion or fragment. In each case, give a likely structure of the ion responsible for the base peak.
(a) base peak at 105
(b) base peak at 72
(c) M+ doublet at 198 and 200, base peak at 91
(d) base peak at 91, large peak at 43