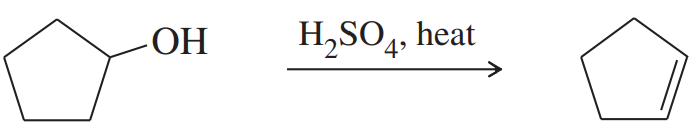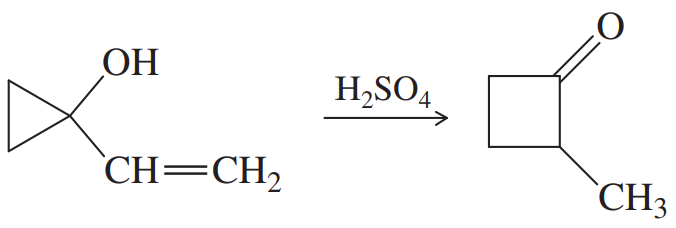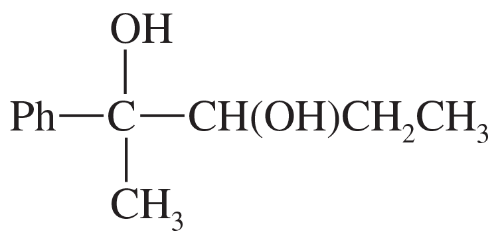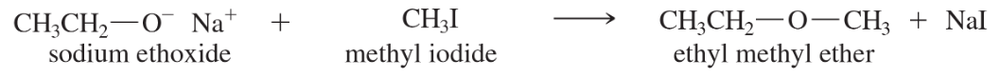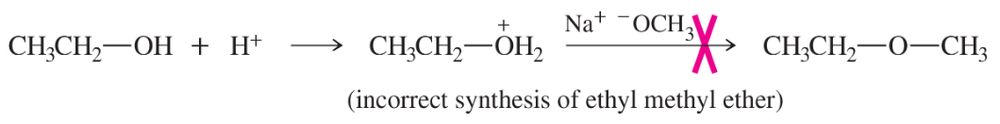Back
BackProblem 16
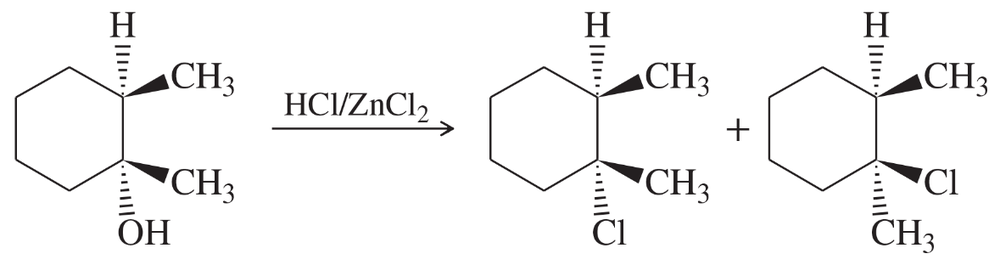
Explain the products observed in the following reaction of an alcohol with the Lucas reagent.
Problem 17
When cis-2-methylcyclohexanol reacts with the Lucas reagent, the major product is 1-chloro-1-methylcyclohexane. Propose a mechanism to explain the formation of this product.
Problem 18
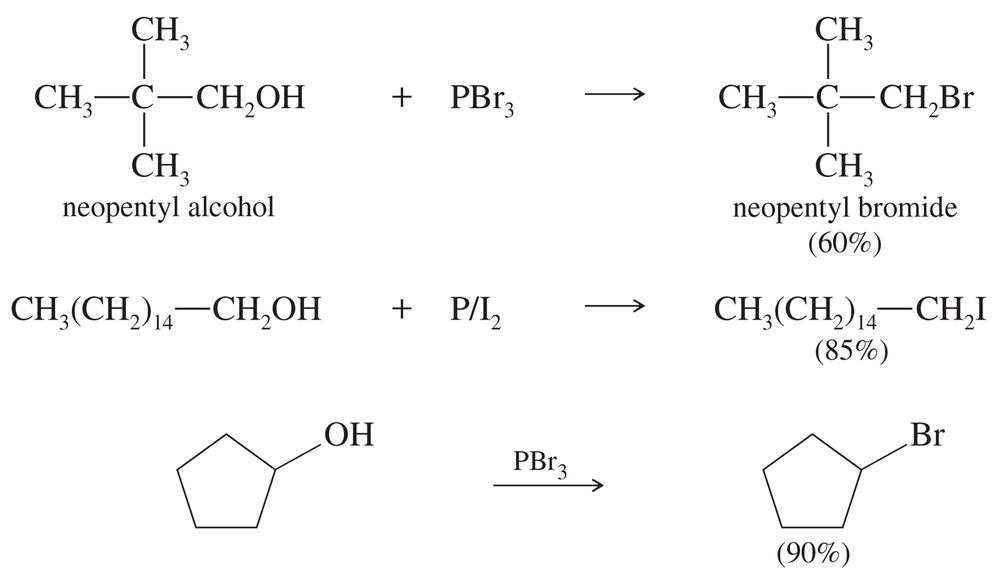
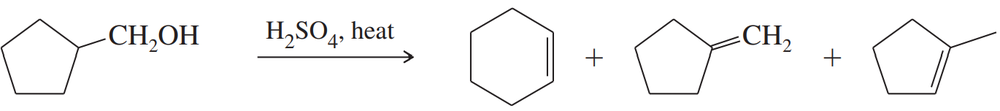
Write balanced equations for the three preceding reactions.
Problem 19
Suggest how you would convert trans-4-methylcyclohexanol to
a. trans-1-chloro-4-methylcyclohexane.
b. cis-1-chloro-4-methylcyclohexane.
Problem 20a,b
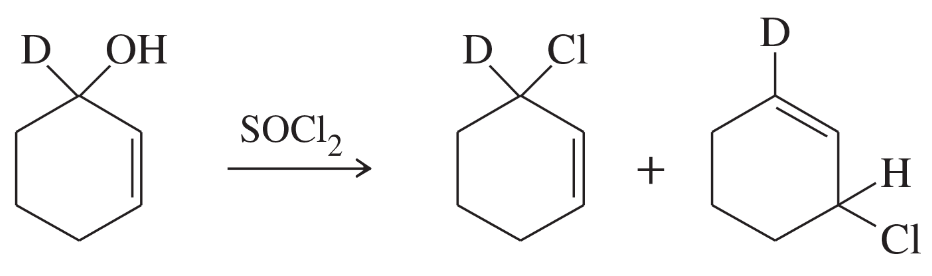
Two products are observed in the following reaction.
a. Suggest a mechanism to explain how these two products are formed.
b. Your mechanism for part (a) should be different from the usual mechanism of the reaction of SOCl2 with alcohols. Explain why the reaction follows a different mechanism in this case.
Problem 21a,b
Give the structures of the products you would expect when each alcohol reacts with (1) HCl, ZnCl2; (2) HBr; (3) PBr3; (4) P/I2; and (5) SOCl2.
(a) butan-1-ol
(b) 2-methylbutan-2-ol
Problem 22d,e
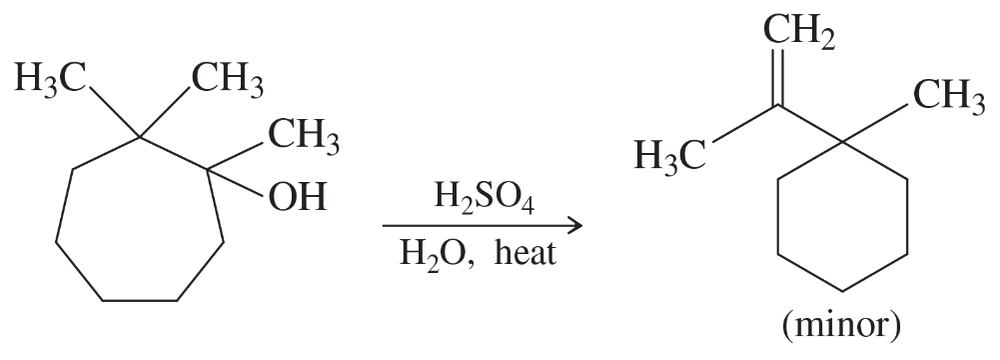
Predict the products of the sulfuric acid-catalyzed dehydration of the following alcohols. When more than one product is expected, label the major and minor products.
(d) 1-isopropylcyclohexanol
(e) 2-methylcyclohexanol
Problem 23
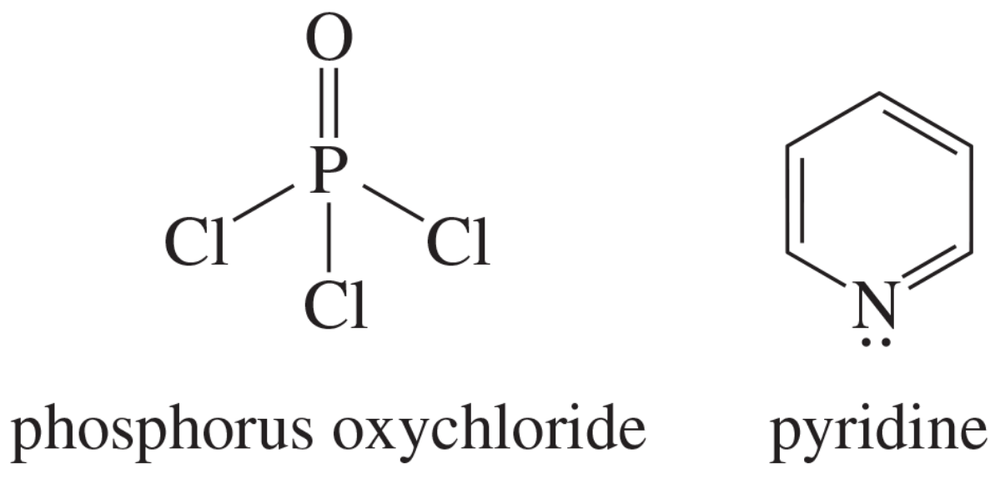
Some alcohols undergo rearrangement or other unwanted side reactions when they dehydrate in acid. Alcohols may be dehydrated under mildly basic conditions using phosphorus oxy-chloride (POCl3) in pyridine. The alcohol reacts with phosphorus oxychloride much like it reacts with tosyl chloride (Section 11-5), displacing a chloride ion from phosphorus to give an alkyl dichlorophosphate ester. The dichlorophosphate group is an outstanding leaving group. Pyridine reacts as a base with the dichlorophosphate ester to give an E2 elimination. Propose a mechanism for the dehydration of cyclohexanol by POCl3 in pyridine.
Problem 24a
Contrast the mechanisms of the two preceding reactions, the dehydration and condensation of ethanol.
Problem 25
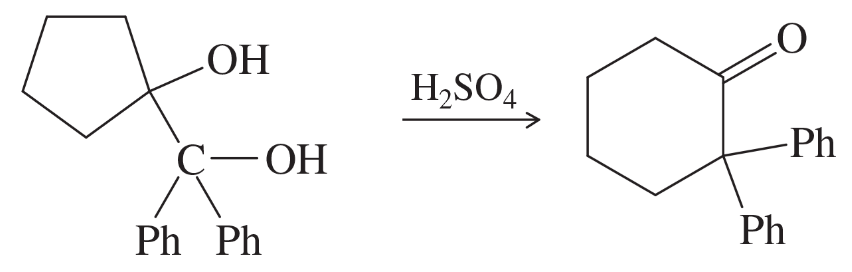
Explain why the acid-catalyzed condensation is a poor method for the synthesis of an unsymmetrical ether such as ethyl methyl ether, CH3CH2-O-CH3.
Problem 26a
Propose a mechanism for each reaction.
(a)
Problem 26c
Propose a mechanism for each reaction.
(c)
Problem 27
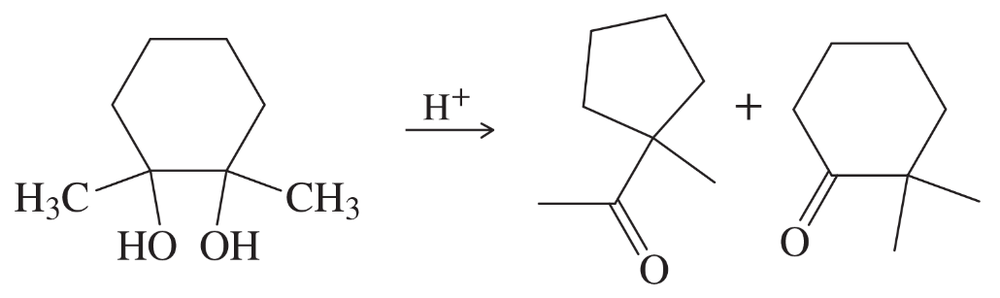
When the following substituted cycloheptanol undergoes dehydration, one of the minor products has undergone a ring contraction. Propose a mechanism to show how this ring contraction occurs.
Problem 28
Propose a mechanism for each reaction.
(a)
(b)
Problem 29
The following reaction involves a starting material with a double bond and a hydroxy group, yet its mechanism resembles a pinacol rearrangement. Propose a mechanism, and point out the part of your mechanism that resembles a pinacol rearrangement.
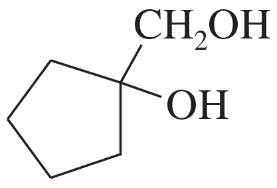
Problem 30a,b
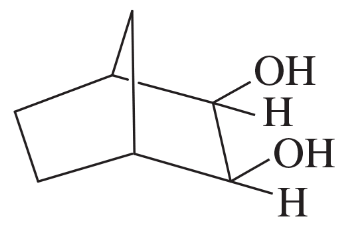
Predict the products formed by periodic acid cleavage of the following diols.
(a) CH3CH(OH)CH(OH)CH3
(b)
Problem 30c,d
Predict the products formed by periodic acid cleavage of the following diols.
(c)
(d)
Problem 31c,d
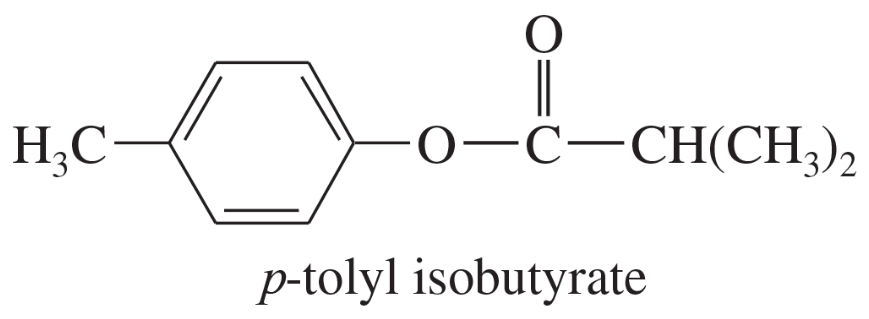
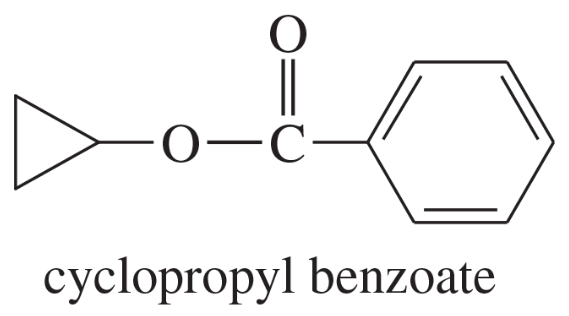
Show the alcohol and the acid chloride that combine to make the following esters.
(c)
(d)
Problem 32
Use resonance forms of the conjugate bases to explain why methanesulfonic acid (CH3SO3H, pKa = –2.6) is a much stronger acid than acetic acid (CH3COOH, pKa = 4.8).
Problem 33
A good Williamson synthesis of ethyl methyl ether would be
What is wrong with the following proposed synthesis of ethyl methyl ether? First, ethanol is treated with acid to protonate the hydroxy group (making it a good leaving group), and then sodium methoxide is added to displace water.
Problem 34
(a) Show how ethanol and cyclohexanol may be used to synthesize cyclohexyl ethyl ether (tosylation followed by the Williamson ether synthesis).
(b) Why can't we synthesize this product simply by mixing the two alcohols, adding some sulfuric acid, and heating?
Problem 35
A student wanted to use the Williamson ether synthesis to make (R)-2-ethoxybutane. He remembered that the Williamson synthesis involves an SN2 displacement, which takes place with inversion of configuration. He ordered a bottle of (S)-butan-2-ol for his chiral starting material. He also remembered that the SN2 goes best on primary halides and tosylates, so he made ethyl tosylate and sodium (S)-but-2-oxide. After warming these reagents together, he obtained an excellent yield of 2-ethoxybutane.
a. What enantiomer of 2-ethoxybutane did he obtain? Explain how this enantiomer results from the SN2 reaction of ethyl tosylate with sodium (S)-but-2-oxide.
b. What would have been the best synthesis of (R)-2-ethoxybutane?
c. How can this student convert the rest of his bottle of (S)-butan-2-ol to (R)-2-ethoxybutane?
Problem 36
Phenols (pKa ≈ 10) are more acidic than other alcohols, so they are easily deprotonated by sodium hydroxide or potassium hydroxide. The anions of phenols (phenoxide ions) can be used in the Williamson ether synthesis, especially with very reactive alkylating reagents such as dimethyl sulfate. Using phenol, dimethyl sulfate, and other necessary reagents, show how you would synthesize methyl phenyl ether.
Problem 37a
To practice working through the early parts of a multistep synthesis, devise syntheses of
(a) pentan-3-one from alcohols containing no more than three carbon atoms.
Problem 37b
To practice working through the early parts of a multistep synthesis, devise syntheses of
(b) 3-ethylpentan-2-one from compounds containing no more than three carbon atoms.
Problem 38a
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(a) trans-cyclopentane-1,2-diol
Problem 38b
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(b) 1-chloro-1-ethylcyclopentane
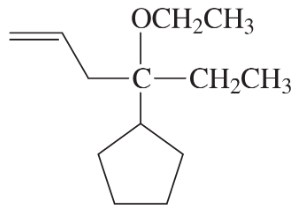
Problem 38c
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(c)
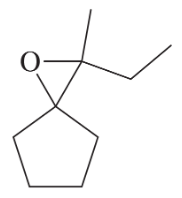
Problem 38d
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(d)
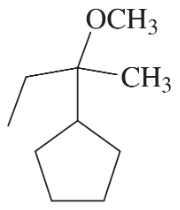
Problem 38e
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(e)