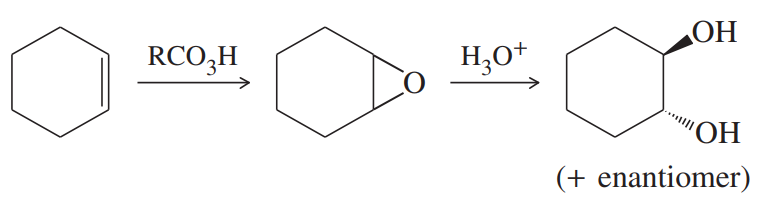
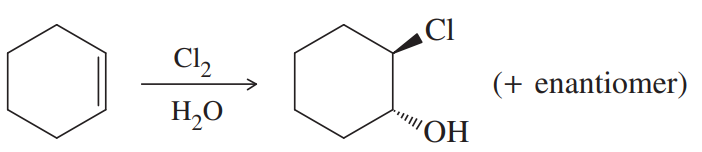
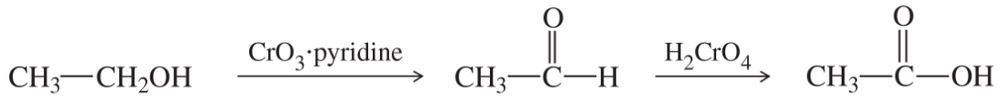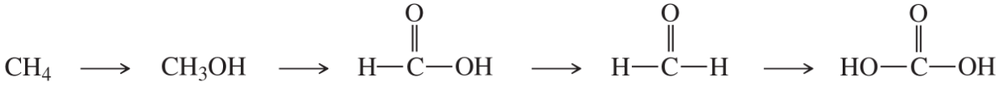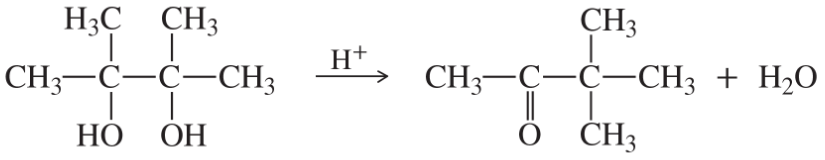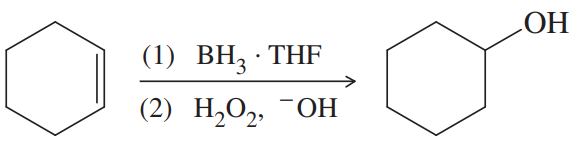Back
BackProblem 1a,b,c
Classify each reaction as an oxidation, a reduction, or neither.
(a)
(b)
(c)
Problem 1d,e,f
Classify each reaction as an oxidation, a reduction, or neither.
(d)
(e)
(f)
Problem 1g,h,i
Classify each reaction as an oxidation, a reduction, or neither.
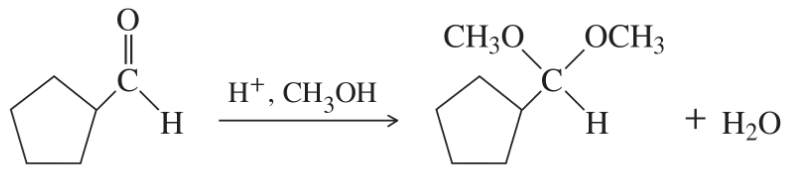
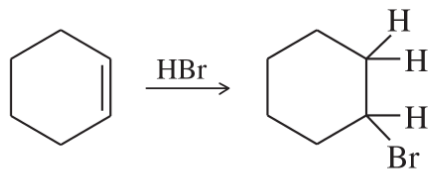
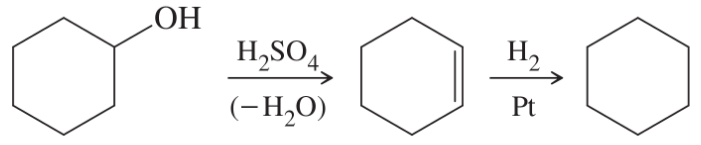
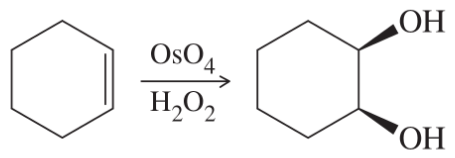
(g)
(h)
(i)
Problem 1j,k,l
Classify each reaction as an oxidation, a reduction, or neither.
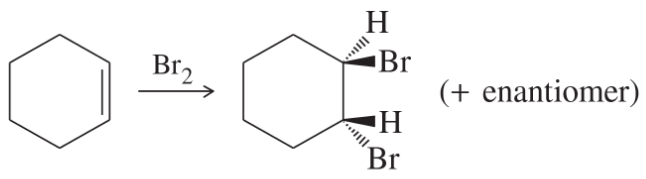
(j)
(k)
(l)
Problem 2a,b,c
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
a. cyclohexanol
b. 1-methylcyclohexanol
c. cyclopentylmethanol
Problem 2d
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
d. cyclohexanone
Problem 2e
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
e. cyclohexane
Problem 2f
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
f. 1-phenylpropan-1-ol
Problem 2g,h
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
g. hexan-1-ol
h. acetaldehyde, CH3CHO
Problem 3a,b,c
We have covered several oxidants that use a multi-valent atom (Cr, Cl, S, or I) as their active species, going from a higher oxidation state before the oxidation to a lower oxidation state after oxidizing the alcohol. Draw the Lewis structures of the following atoms, before and after the oxidation of an alcohol to a ketone or aldehyde. How many bonds to oxygen does each atom have before and after the oxidation?
a. the Cr in chromic acid
b. the Cl in sodium hypochlorite
c. the S in the Swern oxidation
Problem 4a,b
Give the structure of the principal product(s) when each of the following alcohols reacts with (1) Na2Cr2O7/H2SO4, (2) PCC, (3) DMP, and (4) 1 equiv NaOCl-TEMPO.
a. octan-1-ol
b. octan-3-ol
Problem 4c(1,2)
Give the structure of the principal product(s) when each of the following alcohols reacts with (1) Na2Cr2O7/H2SO4, (2) PCC
c. 4-hydroxydecanal
Problem 4c(3,4)
Give the structure of the principal product(s) when each of the following alcohols reacts with (3) DMP, and (4) 1 equiv NaOCl-TEMPO.
c. 4-hydroxydecanal
Problem 4d(1,2)
Give the structure of the principal product(s) when each of the following alcohols reacts with (1) Na2Cr2O7/H2SO4, (2) PCC
d. 1-methylcyclohexan-1,4-diol