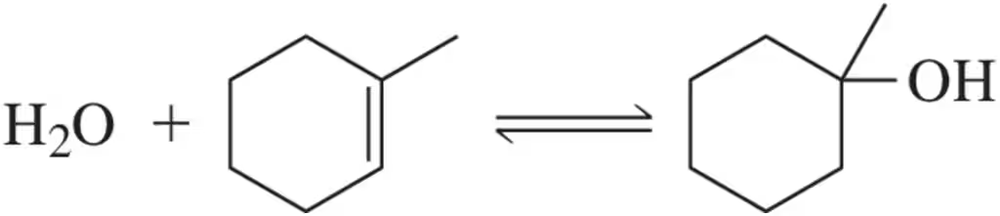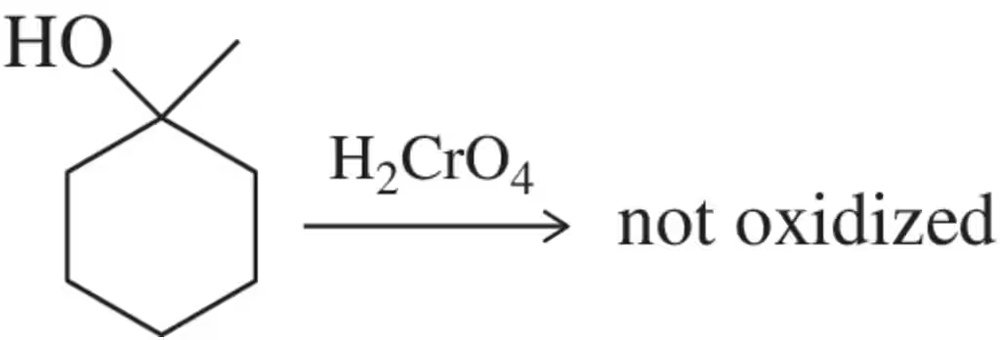Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and Elimination
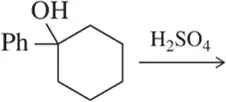
Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and EliminationProblem 38
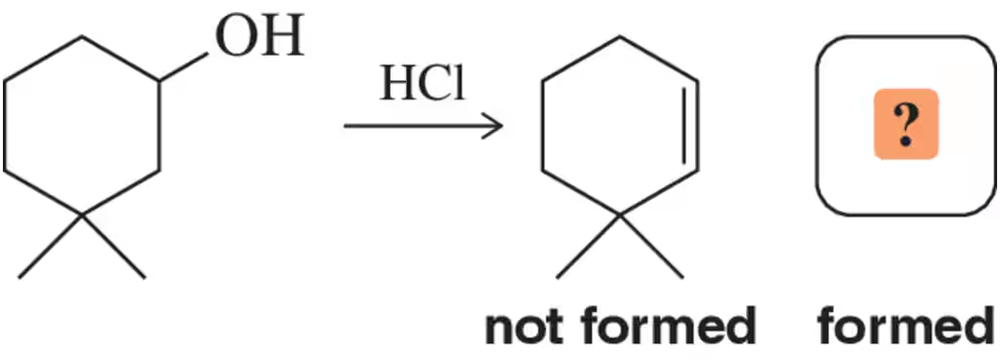
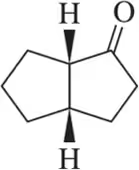
When HCl was used for the attempted dehydration reaction shown, a reaction occurred, but none of the desired product was formed. Suggest the identity of the actual product obtained.
Problem 39
Predict which side of the equilibrium is favored by ∆H, ∆G, and ∆S.
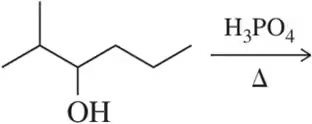
Problem 40a
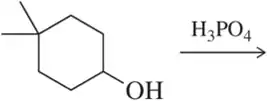
Predict the major product of the following elimination reactions.
(a)
Problem 40b
Predict the major product of the following elimination reactions.
(b)
Problem 40c
Predict the major product of the following elimination reactions.
(c)
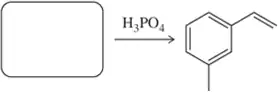
Problem 41a
Identify the alcohol(s) that would produce the following alkenes under the given conditions.
(a)
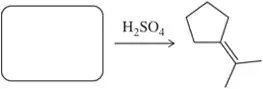
Problem 41b
Identify the alcohol(s) that would produce the following alkenes under the given conditions.
(b)
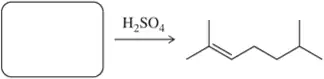
Problem 41c
Identify the alcohol(s) that would produce the following alkenes under the given conditions.
(c)
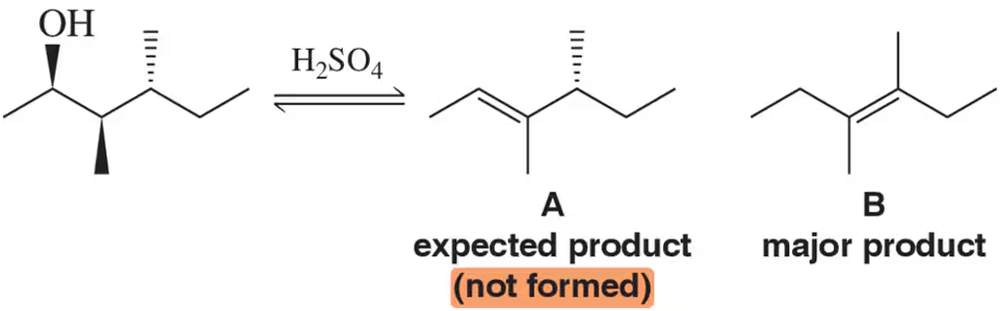
Problem 42
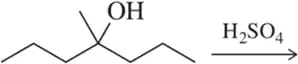
Treatment of the following alcohol was expected to give alkene A. Instead, B was produced as the major product. Suggest a mechanism by which B formed.
Problem 43a
Predict the product(s) of the reactions shown.
(a)
Problem 43b
Predict the product(s) of the reactions shown.
(b)
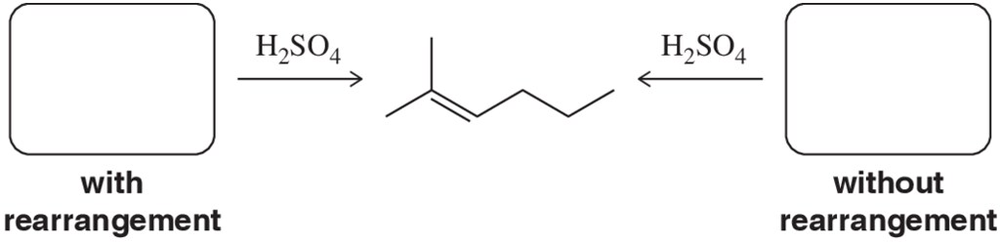
Problem 44a
Identify two different alcohols that can be dehydrated (one with rearrangement) to form the alkene shown.
Problem 44b
Identify two different alcohols that can be dehydrated (one without rearrangement) to form the alkene shown.
Problem 45
Show a reaction coordinate diagram for the two processes in Figure 13.41 that rationalizes pathway B as the one that gives the major product.
<IMAGE>
Problem 46b
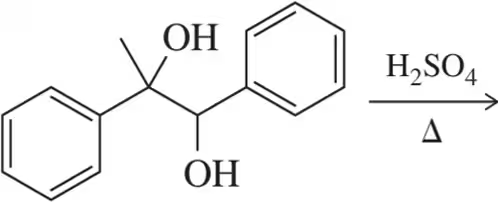
Predict the product of the following pinacol rearrangements.
(b)
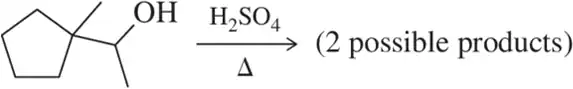
Problem 46c
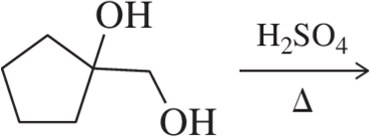
Predict the product of the following pinacol rearrangements.
(c)
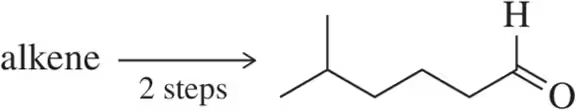
Problem 47c
Suggest an alkene that, in two steps, could be converted into each of the following ketones. Each sequence should involve a pinacol rearrangement.
(c)
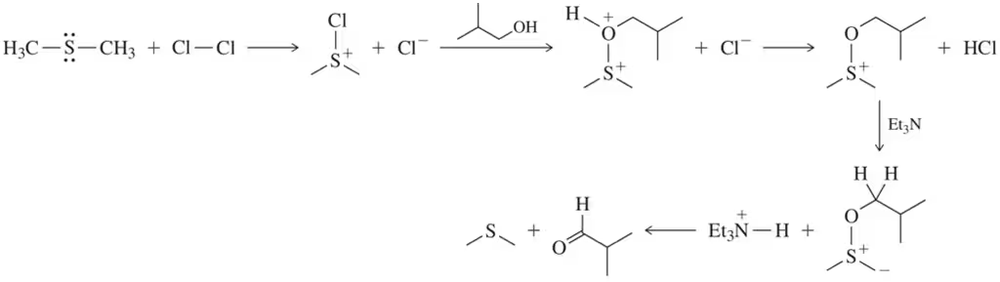
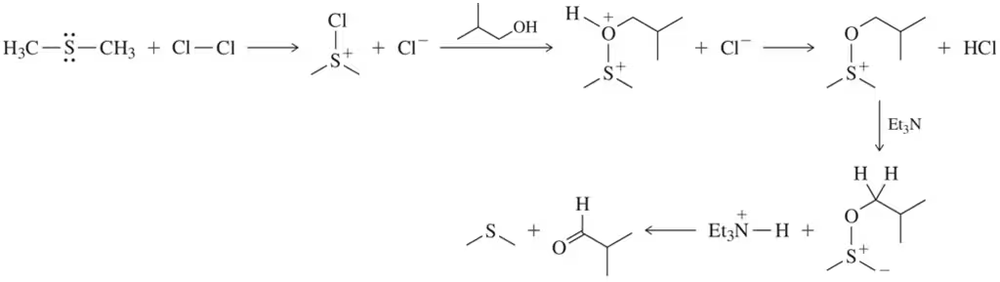
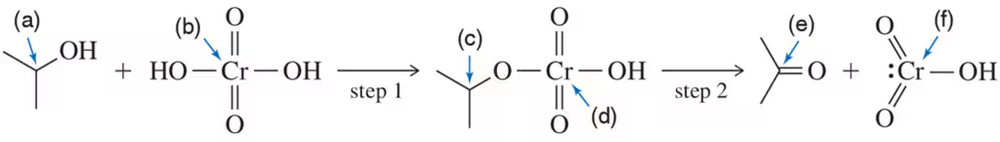
Problem 48
The intermediates for the Swern oxidation, a reaction introduced in Section 13.9.4, are shown. Provide the arrow-pushing mechanism that rationalizes the formation of each intermediate and the final product(s).
Problem 49
In Assessment 13.48, identify the individual step that represents the general mechanism for all alcohol oxidation reactions.
Problem 50
Every oxidation is accompanied by a reduction. Identify the species that is reduced in the Swern oxidation in Assessment 13.48.
Problem 51a
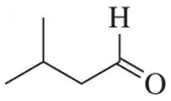
Identify the alcohols that would undergo oxidation to produce the following carbonyl compounds.
(a)
Problem 51b
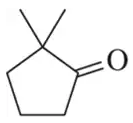
Identify the alcohols that would undergo oxidation to produce the following carbonyl compounds.
(b)
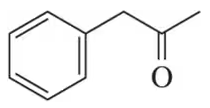
Problem 51c
Identify the alcohols that would undergo oxidation to produce the following carbonyl compounds.
(c)
Problem 51d
Identify the alcohols that would undergo oxidation to produce the following carbonyl compounds.
(d)
Problem 52a
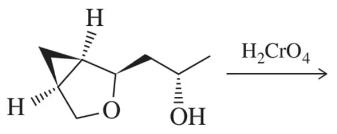
Predict the product of the oxidation reactions shown.
(a)
Problem 52b
Predict the product of the oxidation reactions shown.
(b)
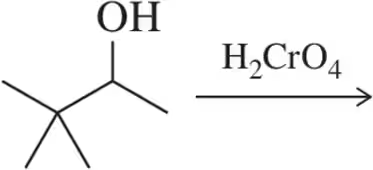
Problem 53e
The intermediates for the oxidation of isopropanol to acetone are shown. Calculate oxidation numbers for the indicated atoms, then use them to determine in which step oxidation occurs.
(e)
Problem 54
Tertiary (3°)alcohols are not oxidized by chromic acid. Why?
Problem 55
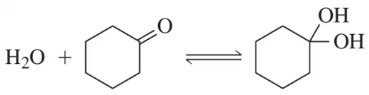
Though ketones, like aldehydes, are in equilibrium with a hydrated form, they cannot be further oxidized. Why?
Problem 56
If there is no water present, the hydrate of an aldehyde cannot form. Could an aldehyde itself (not the hydrate) be oxidized to a carboxylic acid? Why?