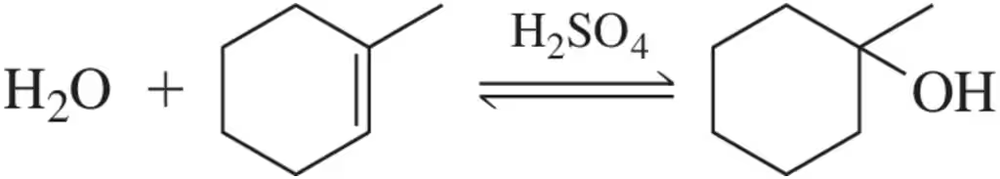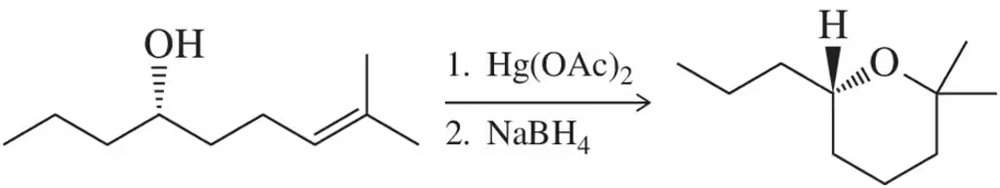Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and Elimination
Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and EliminationProblem 106f(i-xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
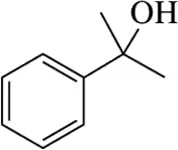
(f)
Problem 106k(vii,viii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions:(vii) HCl; (viii) HBr If no reaction occurs, write 'no reaction.'
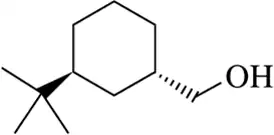
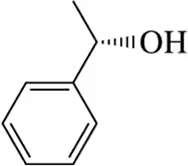
(k)
Problem 106k(i,ii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃. If no reaction occurs, write 'no reaction.'
(k)
Problem 106k(xi,xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (xi) HOCl, H2O (xii) HIO4 If no reaction occurs, write 'no reaction.'
(k)
Problem 106k(iii,iv)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (iii) SOCl₂ , NEt₃, and (iv) 1. TsCl, Et₃N 2. NaCN. If no reaction occurs, write 'no reaction.'
(k)
Problem 106k(v,vi)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ If no reaction occurs, write 'no reaction.'
(k)
Problem 106k(ix,x)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (ix) PCC; (x) H2CrO4 , H2O If no reaction occurs, write 'no reaction.'
(k)
Problem 106o(vii,viii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vii) HCl; (viii) HBr; If no reaction occurs, write 'no reaction.'
(o)
Problem 106o(ix,x)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (ix) PCC; (x) H₂CrO₄ , H₂O. If no reaction occurs, write 'no reaction.'
(o)
Problem 106o(iii,iv)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; If no reaction occurs, write 'no reaction.'
(o)
Problem 106o(xi,xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(o)
Problem 106o(i-xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(o)
Problem 106o(v,vi)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ If no reaction occurs, write 'no reaction.'
(o)
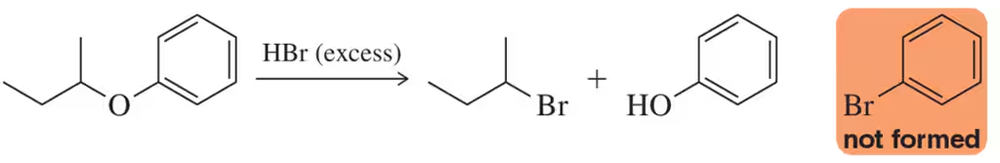
Problem 107
Cleavage of the following ether produces the alcohol and haloalkane only, regardless of how much HBr is used. Thinking about the mechanism of the reaction, explain why bromobenzene is not also a product of this reaction.
Problem 109
Triphenylphosphine and iodine can be used to convert alcohols to iodoalkanes. Suggest a mechanism for this reaction. [Triphenylphosphine first acts as a nucleophile in this reaction.]
Problem 110
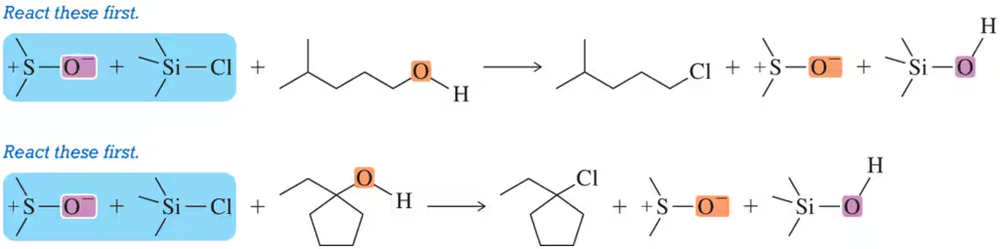
Another method for converting alcohols to chloroalkanes makes use of chlorotrimethylsilane (TMSCl) and DMSO. Suggest a mechanism for this reaction to form (a) a 1° chloroalkane and (b) a 3° chloroalkane. [The reaction begins by the reaction of DMSO and TMSCl and is analogous to the Swern oxidation.]
Problem 111
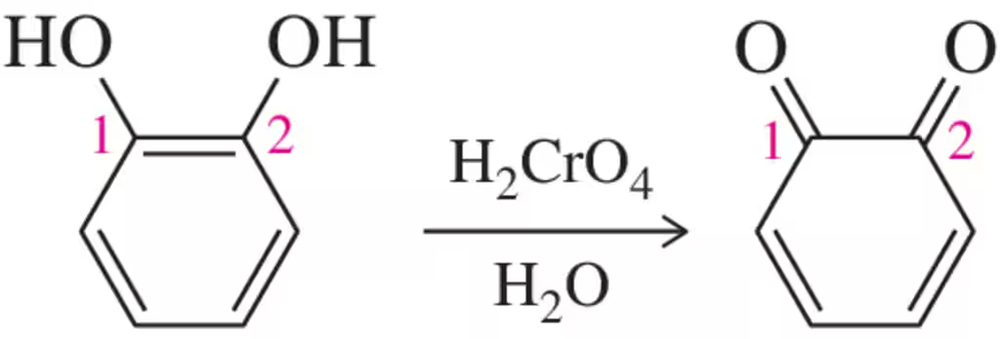
We explain in Chapter 24 that bisphenols can be oxidized to quinones.
(a) Calculate the oxidation numbers of C1 and C₂ in going from reactant to product.
(b) Provide a mechanism for this transformation. [The reaction begins like the alcohol oxidations of Section 13.9.]
Problem 112
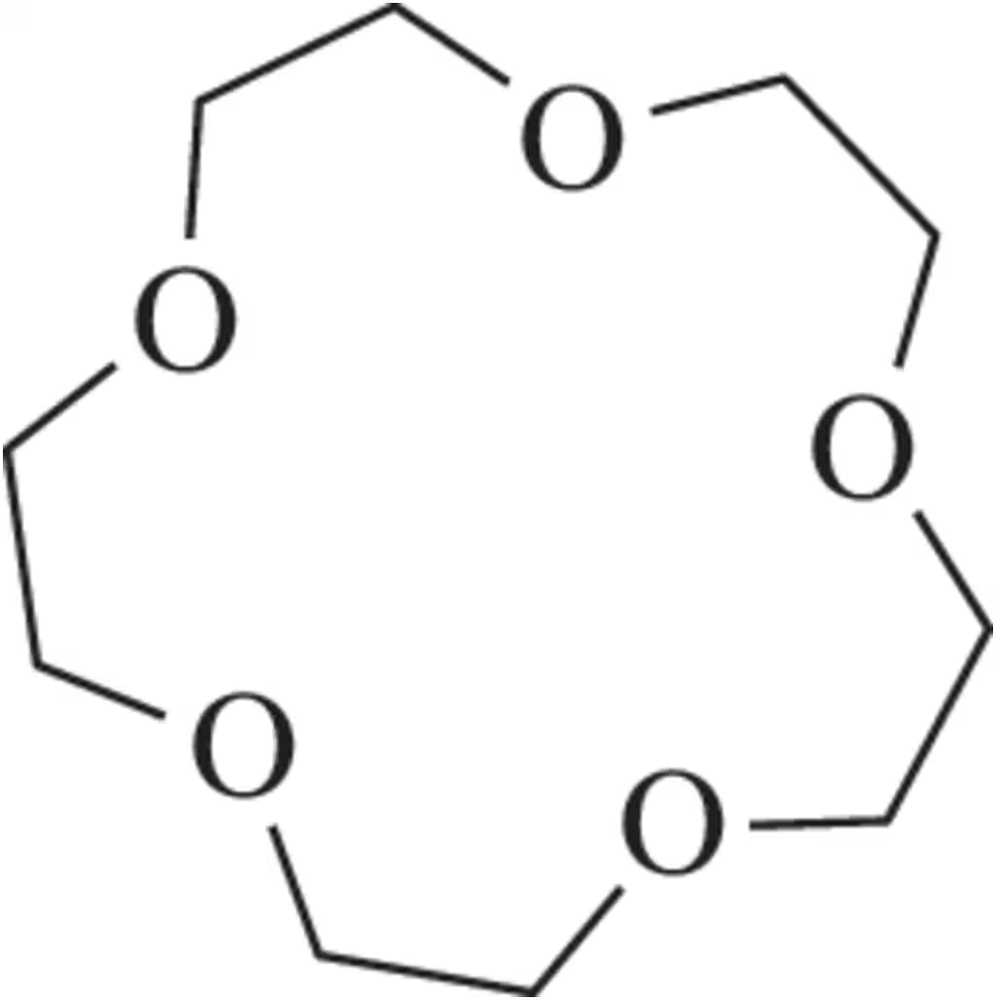
In Chapter 12, we learned that crown ethers were used to increase the rate of SN2 reactions (Assessment 12.80). Suggest a synthesis of 15-crown-5 using the reactions learned here in Chapter 13.
Problem 113
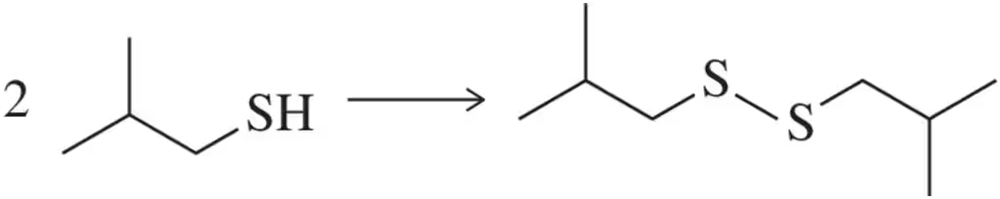
Thiols are prone to dimerize through the formation of a disulfide bond in a reaction that stylists use to induce permanent curls in hair. What type of reagent would you use to effect this transformation?
Problem 114
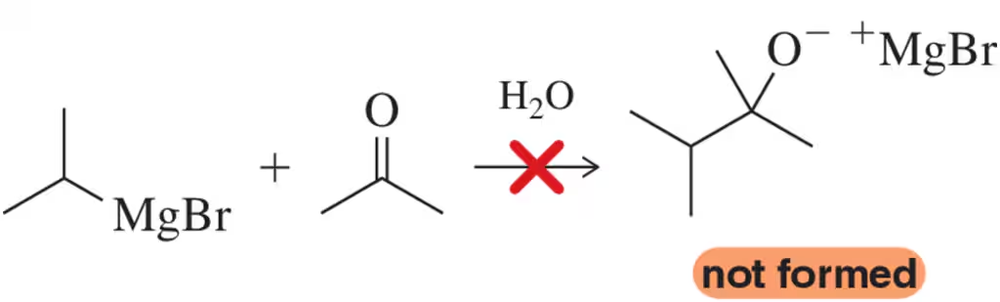
A chemist attempted the reaction below, one we introduce in Chapter 17, expecting the reaction between a strong nucleophile and a ketone in water to give an alkoxide product.
(a) Why did the reaction fail?
(b) How could the reaction conditions be changed to give a successful reaction?
Problem 115a
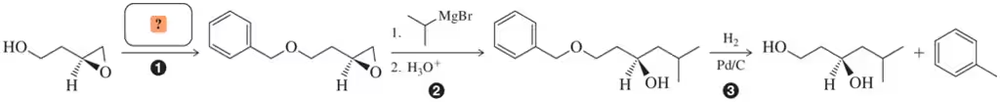
Benzyl ethers make excellent protecting groups according to the general scheme shown here.
(a) How would you protect the 1° alcohol as a benzyl ether in the first step?
Problem 116
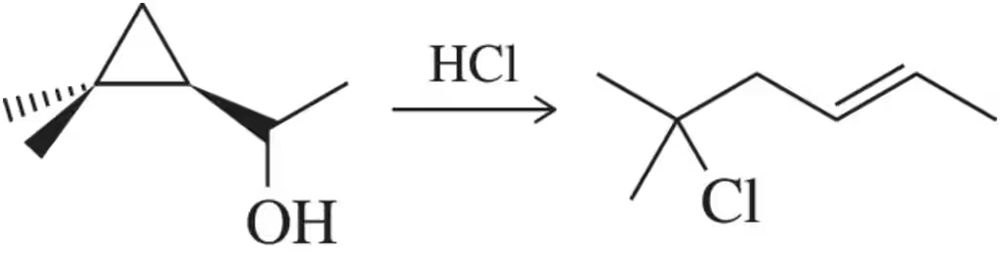
Suggest a mechanism for the following substitution reaction.
Problem 117a
The acid-catalyzed dehydration we learned in this chapter is reversible, as shown below.
(a) Propose a mechanism for the formation of an alcohol from an alkene.
Problem 117b
The acid-catalyzed dehydration we learned in this chapter is reversible, as shown below.
(b) Without looking back, how do you know this mechanism is correct?
Problem 117c
The acid-catalyzed dehydration we learned in this chapter is reversible, as shown below.
(c) Which side of the reaction would be favored by running the reaction at low temperatures?
Problem 117d
The acid-catalyzed dehydration we learned in this chapter is reversible, as shown below.
(d) How might you shift the equilibrium to the right?
Problem 118
Assessment 8.74 revealed that oxymercuration could be used to make cyclic esters. Suggest a likely mechanism for this transformation.
Problem 119
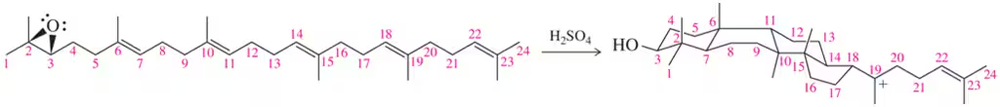
Chapter 8 discussed the synthesis of cholesterol, which proceeds by a cationic cyclization cascade. Without looking back, suggest a mechanism by which the following reaction occurs. [The carbons have been numbered for you.]
Problem 120
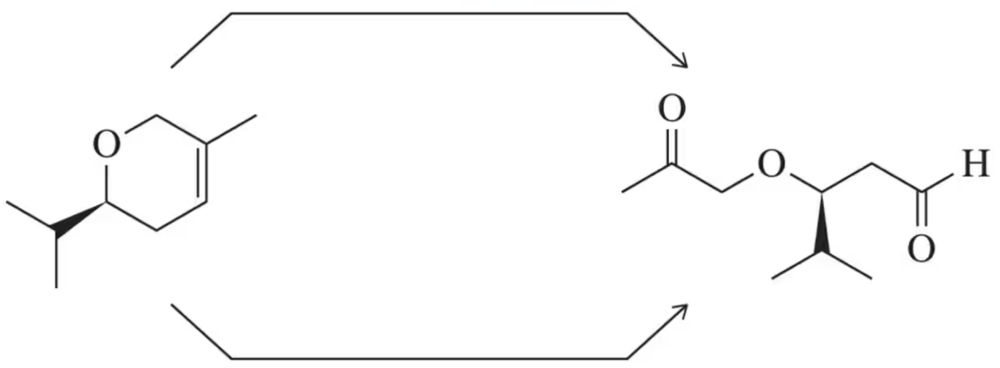
Suggest reagents to carry out the following transformation.