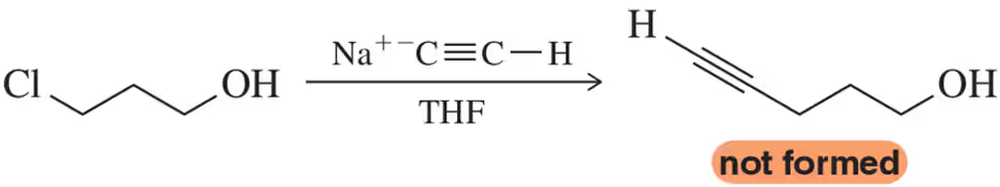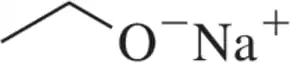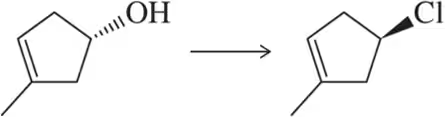Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and Elimination
Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and EliminationProblem 17
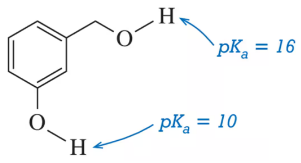
Rationalize the difference in pKₐ values for the two hydroxyl groups.
Problem 18
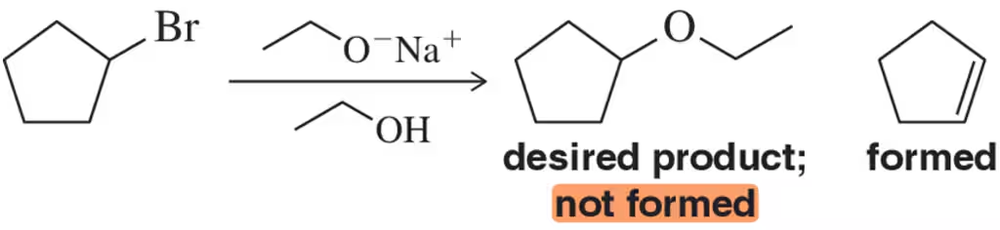
When a secondary haloalkane is treated with sodium ethoxide in ethanol, we predict alkene formation over ether formation. How did we make this determination?
Problem 19
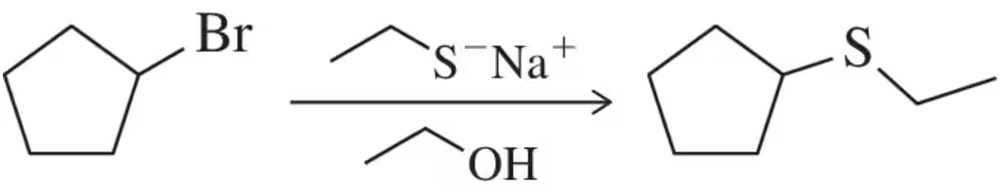
In contrast to the results of Assessment 13.18, when a secondary haloalkane is treated with sodium ethanethiolate, we predict formation of a thioether. How is this rationalized?
Problem 20
The intended SN2 displacement of the 1° chloride by acetylide is unsuccessful for the molecule below. Why?
Problem 21a
Which of the following bases would favorably deprotonate a hydroxyl group?
(a)
Problem 21b
Which of the following bases would favorably deprotonate a hydroxyl group?
(b) NaOH
Problem 21c
Which of the following bases would favorably deprotonate a hydroxyl group?
(c) NaCN
Problem 21d
Which of the following bases would favorably deprotonate a hydroxyl group?
(d) Et3N
Problem 21e
Which of the following bases would favorably deprotonate a hydroxyl group?
(e)
Problem 22
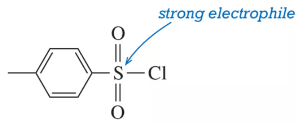
The sulfur atom in toluene sulfonyl chloride (TsCl) is strongly electrophilic. Why?
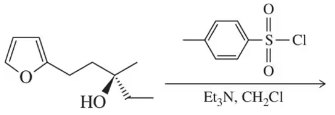
Problem 24a
Predict the product of the following sulfonylation reactions.
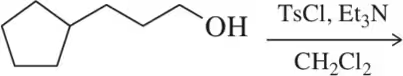
(a)
Problem 24b
Predict the product of the following sulfonylation reactions.
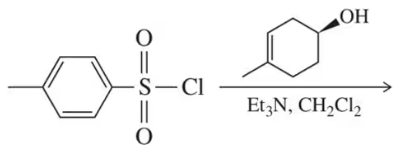
(b)
Problem 24c
Predict the product of the following sulfonylation reactions.
(c)
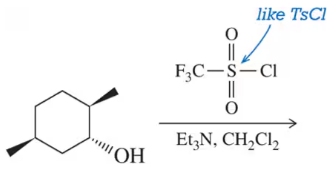
Problem 24d
Predict the product of the following sulfonylation reactions.
(d)
Problem 25
Predict the major product(s) of each of the following reactions.
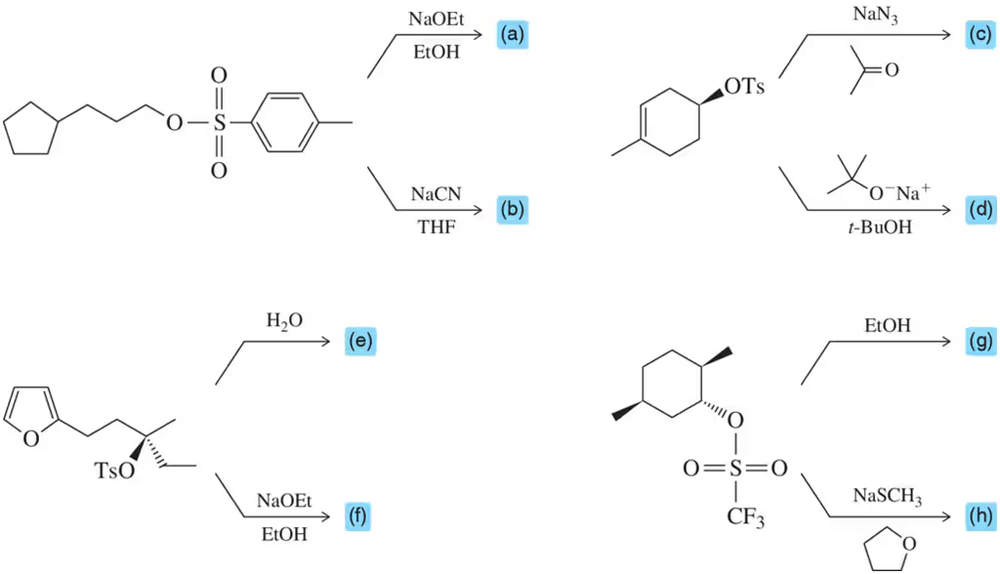
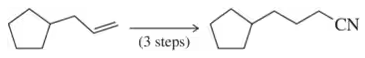
Problem 26a
Complete the following multistep syntheses using tosylate formation as one of the steps. The optimum number of steps for each synthesis is shown.
(a)
Problem 26b
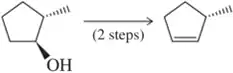
Complete the following multistep syntheses using tosylate formation as one of the steps. The optimum number of steps for each synthesis is shown.
(b)
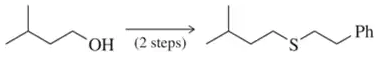
Problem 26c
Complete the following multistep syntheses using tosylate formation as one of the steps. The optimum number of steps for each synthesis is shown.
(c)
Problem 27
On the reaction coordinate diagram for the disfavored nucleophilic displacement of hydroxide, predict the curve that would demonstrate how using a tosylate makes the substitution favorable.
<IMAGE>
Problem 28a
Predict the product of the following reactions.
(a)
Problem 28b
Predict the product of the following reactions.
(b)
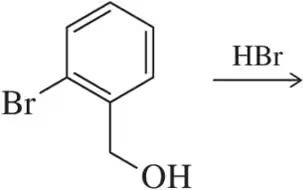
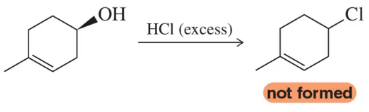
Problem 29
Using an excess of HCl in the following reaction resulted in a product that was not simply the substitution of chlorine for the hydroxyl group. Predict the identity of the product obtained.
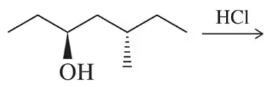
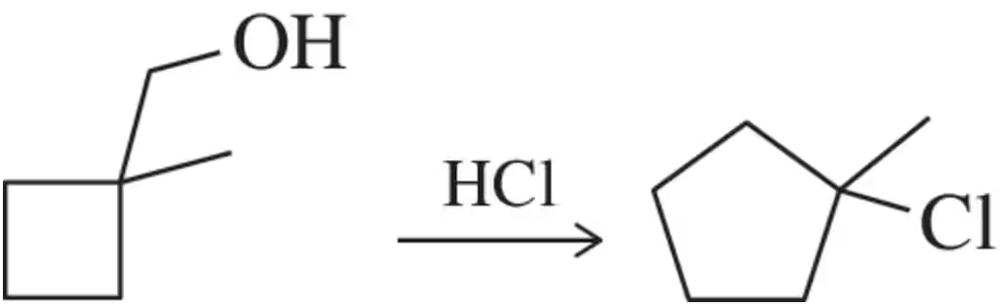
Problem 30
Provide an arrow-pushing mechanism that rationalizes the outcome of the reaction shown.
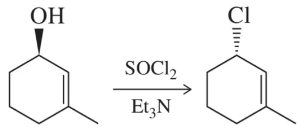
Problem 32
When SOCl2 is used in place of HCl, only one product results. Why are these conditions better?
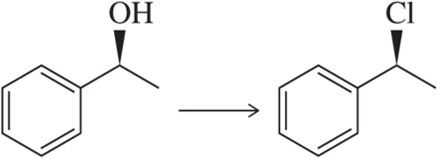
Problem 34a
Identify the reagent that should be used to obtain each stereochemical outcome shown.
(a)
Problem 34b
Identify the reagent that should be used to obtain each stereochemical outcome shown.
(b)
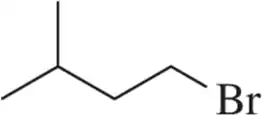
Problem 35a
Provide the alcohol that would be used to make the bromoalkanes shown using PBr₃.
(a)
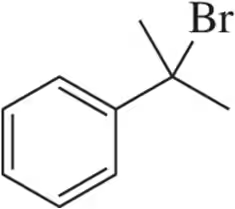
Problem 35b
Provide the alcohol that would be used to make the bromoalkanes shown using PBr₃.
(b)
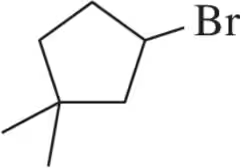
Problem 35c
Provide the alcohol that would be used to make the bromoalkanes shown using PBr₃.
(c)
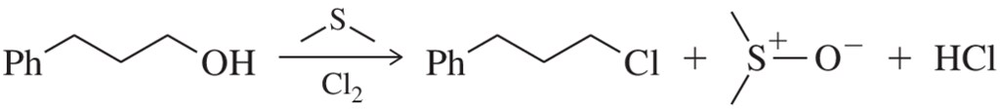
Problem 36
Besides PBr3. and SOCl2 , there are other ways of synthesizing haloalkanes. One such way is shown. Provide an arrow-pushing mechanism that rationalizes formation of the chloroalkane. [Hint: Dimethyl sulfide is a good nucleophile and Cl₂ is an electrophile. Start by reacting those two together.]