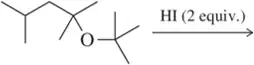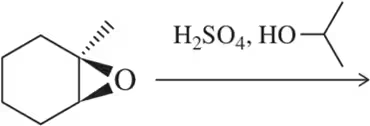Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and Elimination
Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and EliminationProblem 73a
Identify the alkene and alcohol partners that could be used to make the following ethers.
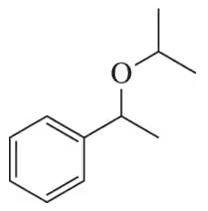
(a)
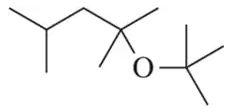
Problem 73c
Identify the alkene and alcohol partners that could be used to make the following ethers.
(c)
Problem 74a
Predict the products of the following reactions.
(a)
Problem 74b
Predict the products of the following reactions.
(b)
Problem 74c
Predict the products of the following reactions.
(c)
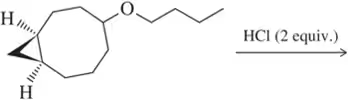
Problem 75
Show an arrow-pushing mechanism that rationalizes formation of the two products. Which C―O bond will break first? Why?
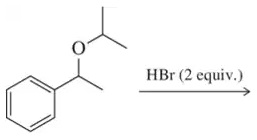
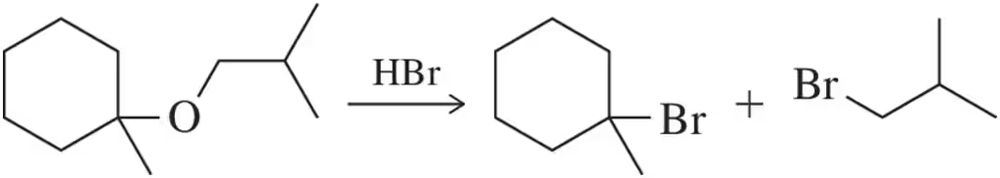
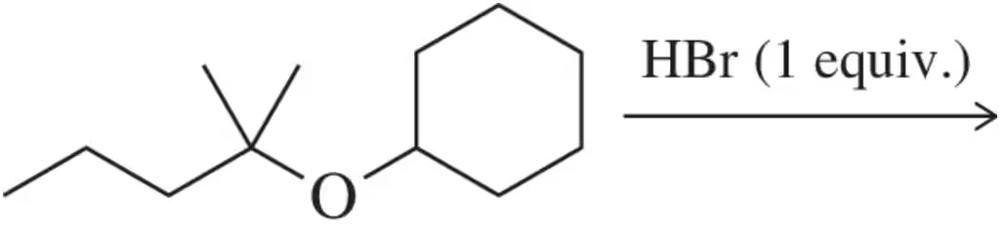
Problem 76
Predict the alcohol and haloalkane that will form upon reaction of the ether shown with one equivalent of HBr. [Hint: Think carefully about which side will become the halide.]
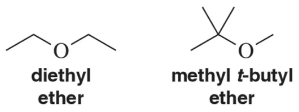
Problem 77
Methyl t-butyl ether (MTBE) is used preferentially over diethyl ether because it is less prone to form peroxides. Explain this observation in terms of the two structures.
Problem 78a
Predict the product of the following reactions.
(a)
Problem 80a
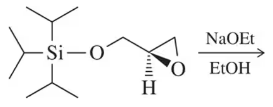
Predict the product of the following epoxide addition reactions.
(a)
Problem 80b
Predict the product of the following epoxide addition reactions.
(b)
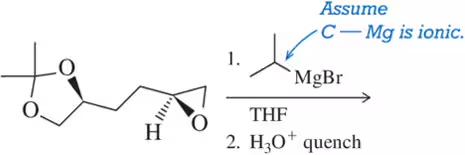
Problem 81b
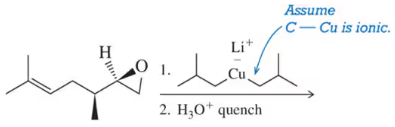
A variety of organometallics, which as strong nucleophiles can react with epoxides, are introduced in Chapter 16. Predict the product of these reactions. [Hint: Assume the carbon–metal bond in each is ionic, with the carbon possessing the negative charge.]
(b)
Problem 81c
A variety of organometallics, which as strong nucleophiles can react with epoxides, are introduced in Chapter 16. Predict the product of these reactions. [Hint: Assume the carbon–metal bond in each is ionic, with the carbon possessing the negative charge.]
(c)
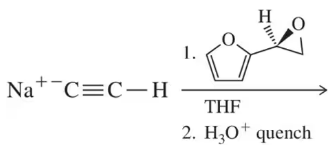
Problem 83
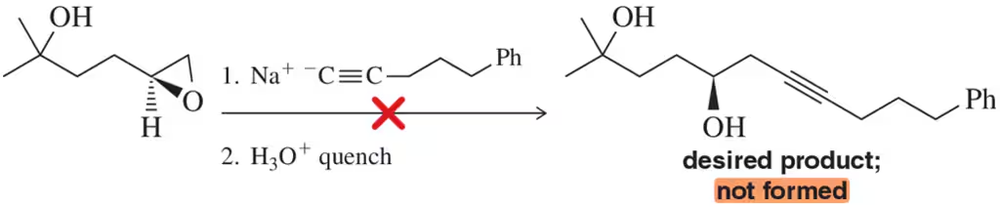
Reaction of the acetylide with the epoxide shown will not form the desired product. What side reaction occurs instead? Why?
Problem 84a
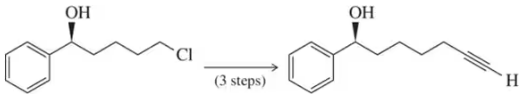
Show how a protecting group might be used to make these reactions successful.
(a)
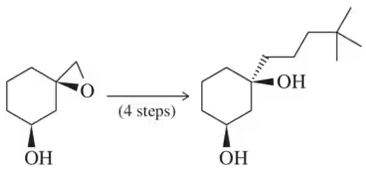
Problem 84b
Show how a protecting group might be used to make these reactions successful.
(b)
Problem 86b
Using IUPAC rules, name the following molecules.
(b)
Problem 86d
Using IUPAC rules, name the following molecules.
(d)
Problem 86e
Using IUPAC rules, name the following molecules.
(e)
Problem 87a
Draw the correct structure from the following IUPAC names:
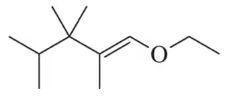
(a) (4R,2Z)-4-methylhex-2-en-1-ol
Problem 87b
Draw the correct structure from the following IUPAC names:
(b) 4-methoxybut-1-yne
Problem 87c
Draw the correct structure from the following IUPAC names:
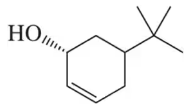
(c) (1S,4R)-4-bromocyclohex-2-en-1-ol
Problem 87d
Draw the correct structure from the following IUPAC names:
(d) (2R,3S)-methoxy-3-methylpentane
Problem 87e
Draw the correct structure from the following IUPAC names:
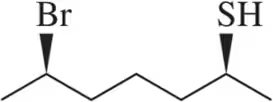
(e) (R)-2,2-dimethyl-1-phenylpropane-1-thiol
Problem 87f
Draw the correct structure from the following IUPAC names:
(f) (S)-4-isopropoxypent-2-yne.
Problem 88a(i)
Predict the product(s) that would result when the following molecules are allowed to react under the following conditions: (i) 1. BH3 2. NaOH, H2O2. If there is no reaction, write 'no reaction.'
(a)
Problem 88a(ii)
Predict the product(s) that would result when the following molecules are allowed to react under the following conditions: (ii) 1. Hg(OAc)2 2. NaBH4.If there is no reaction, write 'no reaction.'
(a)
Problem 88a(iii)
Predict the product(s) that would result when the following molecules are allowed to react under the following conditions: (iii) H2SO4 , H2O . If there is no reaction, write 'no reaction.'
(a)
Problem 88a(iv)
Predict the product(s) that would result when the following molecules are allowed to react under the following conditions: (iv) 1. OsO4 2. NaHSO3. If there is no reaction, write 'no reaction.'
(a)
Problem 88a(v)
Predict the product(s) that would result when the following molecules are allowed to react under the following conditions: (v) H2O. If there is no reaction, write 'no reaction.'
(a)