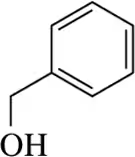
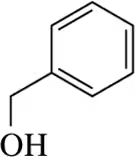
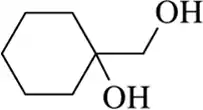
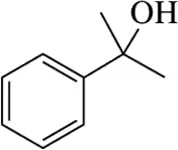
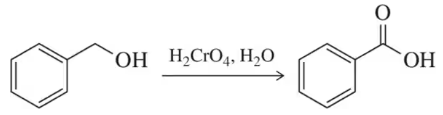Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and Elimination
Ch. 13 - Alcohols, Ethers and Related Compounds: Substitution and EliminationProblem 100b(ii)
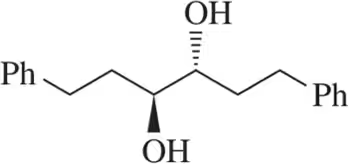
Draw the mechanism for the acid-catalyzed pinacol rearrangement of the following diols.
(b)
Problem 100c
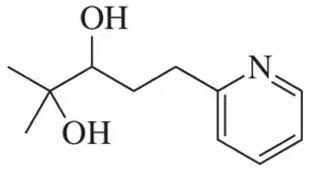
Predict the product and draw the mechanism for the acid-catalyzed pinacol rearrangement of the following diols.
(c)
Problem 101a
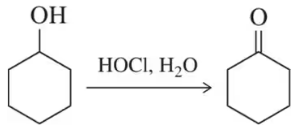
Draw a mechanism for the following oxidation reactions.
(a)
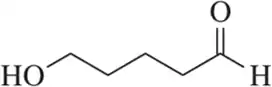
Problem 101b
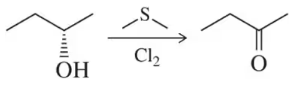
Draw a mechanism for the following oxidation reactions.
(b)
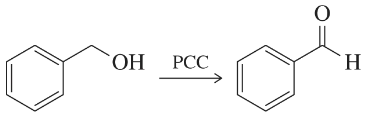
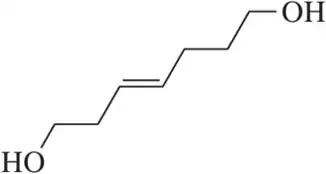
Problem 101c
Draw a mechanism for the following oxidation reactions.
(c)
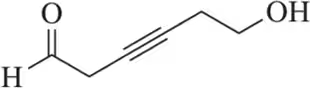
Problem 101d
Draw a mechanism for the following oxidation reactions.
(d)
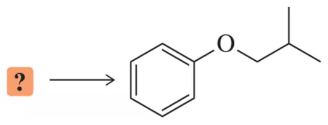
Problem 102
Identify the two possible combinations of haloalkane and alkoxide that can be used to make the following ether.
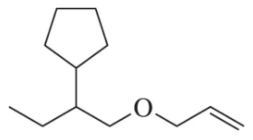
Problem 103
In contrast to Assessment 13.102, only one combination of haloalkane and alkoxide can be used in the Williamson ether synthesis to make the ether shown. Identify the combination and explain why it is the only combination that works.
Problem 104a
Suggest a synthesis for the following molecules beginning with organic molecules containing three or fewer carbons. [You will need to use a protecting group in these syntheses.]
(a)
Problem 104b
Suggest a synthesis for the following molecules beginning with organic molecules containing three or fewer carbons. [You will need to use a protecting group in these syntheses.]
(b)
Problem 104c
Suggest a synthesis for the following molecules beginning with organic molecules containing three or fewer carbons. [You will need to use a protecting group in these syntheses.]
(c)
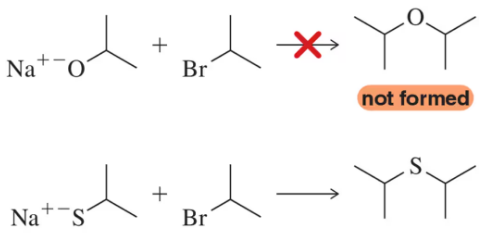
Problem 105
The reaction of alkoxides with haloalkanes is not a viable way to form ethers. (a) Why? (b) Why can thioethers be formed by an analogous reaction?
Problem 106a(i,ii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃. If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(iii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (iii) SOCl2 , NEt3. If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(iv,v)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (iv) 1. TsCl, Et₃N 2. NaCN ; (v) 1. TsCl, Et₃N 2. NaOt-Bu . If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(vi)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vi) H₂SO₄. If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(vii,viii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vii) HCl; (viii) HBr. If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(ix,x)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (ix) PCC; (x) H₂CrO₄ , H₂O. If no reaction occurs, write 'no reaction.'
(a)
Problem 106a(xi,xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (xi) HOCl, H₂O (xii) HIO₄. If no reaction occurs, write 'no reaction.'
(a)
Problem 106c(i,ii-xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(i-xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(i,iii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (iii) SOCl₂, NEt₃. If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(vi)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vi) H₂SO₄. If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(xi,xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(ii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (ii) PBr₃ If no reaction occurs, write 'no reaction.'
(c)
Problem 106c(vii,viii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vii) HCl; (viii) HBr; If no reaction occurs, write 'no reaction.'
(c)
Problem 106f(i-xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(f)
Problem 106f(xi,xii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(f)
Problem 106f(ix,x)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (ix) PCC; (x) H₂CrO₄ , H₂O If no reaction occurs, write 'no reaction.'
(f)
Problem 106f(vii,viii)
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (vii) HCl; (viii) HBr; If no reaction occurs, write 'no reaction.'
(f)