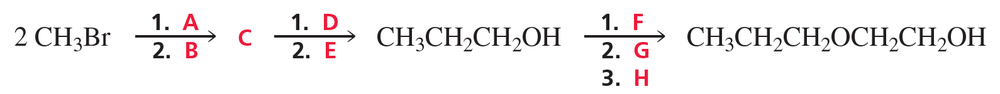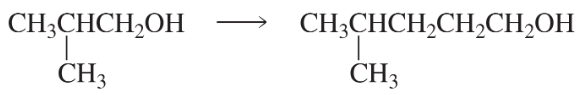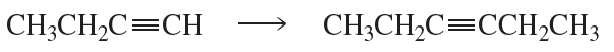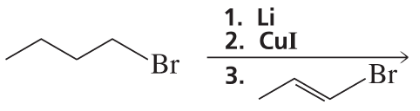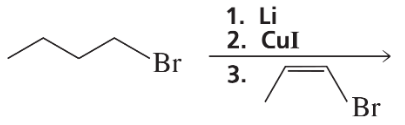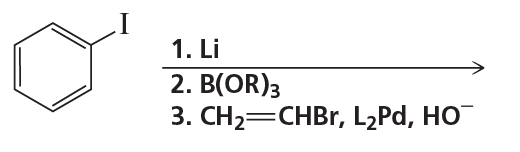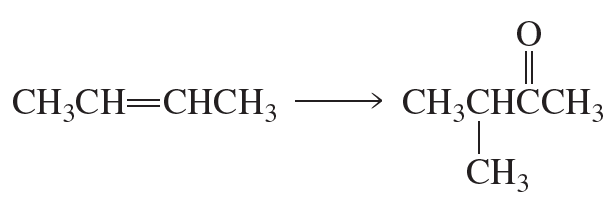Back
BackProblem 29
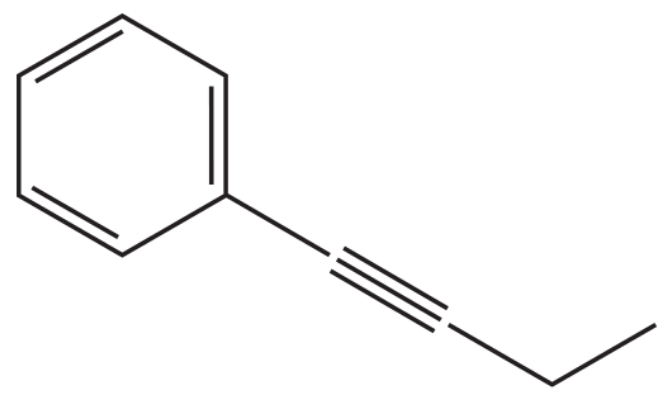
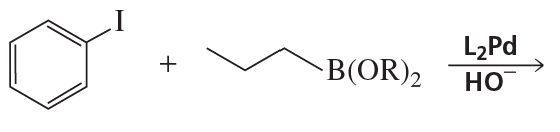
The coupling of an alkyne with an aryl halide in the presence of a palladium catalyst and triethylamine is called a Sonogashira reaction. What reactants couple to form this product?
Problem 30
Identify A through H
Problem 31a
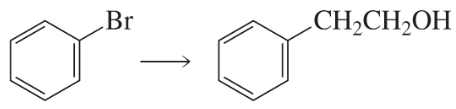
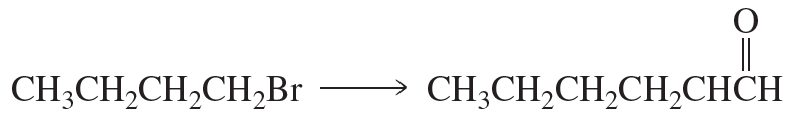
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
a.
Problem 31b
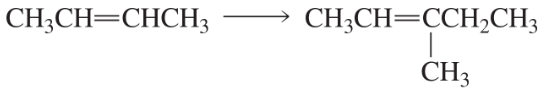
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
b.
Problem 31c
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
c.
Problem 31d
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
d.
Problem 31e
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
e.
Problem 31f
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than three carbons, indicate how each of the following compounds can be prepared:
f.
Problem 32a,b
What alkyl halide reacts with lithium divinylcuprate [(CH2=CH)2CuLi] for the synthesis of each of the following compounds?
a.
b.
Problem 32c,d
What alkyl halide reacts with lithium divinylcuprate [(CH2=CH)2CuLi] for the synthesis of each of the following compounds?
c.
d.
Problem 33a,b
What are the products of the following reactions?
a.
b.
Problem 34c
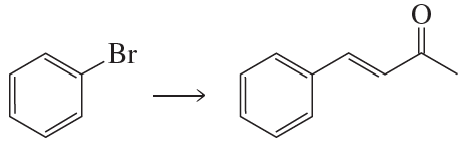
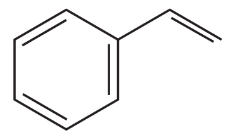
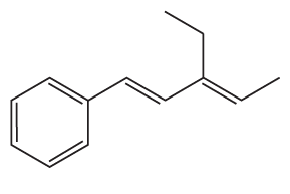
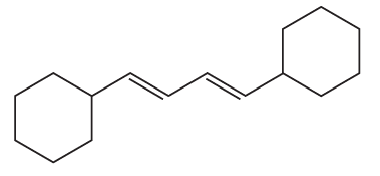
What vinylic halide couples with styrene (C6H5-CH=CH2) in order to synthesize each of the following compounds using a Heck reaction?
c.
Problem 36a
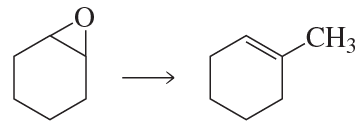
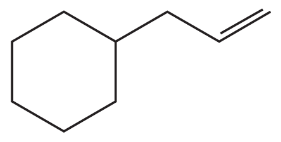
Using ethynylcyclohexane as a starting material and any other needed reagents, how can the following compounds be synthesized?
a.
Problem 37b
What are the products of the following reactions?
b.
Problem 37d
What are the products of the following reactions?
d.
Problem 38a
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than two carbons, indicate how each of the following compounds can be prepared:
a.
Problem 38b
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than two carbons, indicate how each of the following compounds can be prepared:
b.
Problem 38c
Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing compounds with no more than two carbons, indicate how each of the following compounds can be prepared:
c.
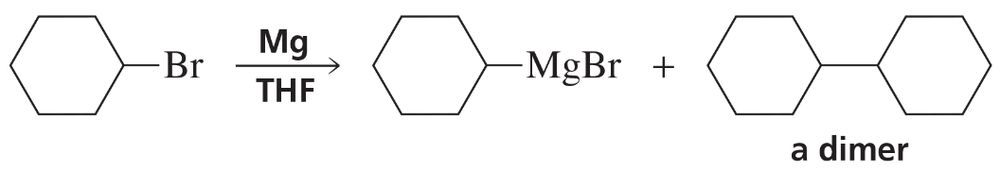
Problem 39
Dimerization is a side reaction that occurs during the preparation of a Grignard reagent. Propose a mechanism that accounts for the formation of the dimer.
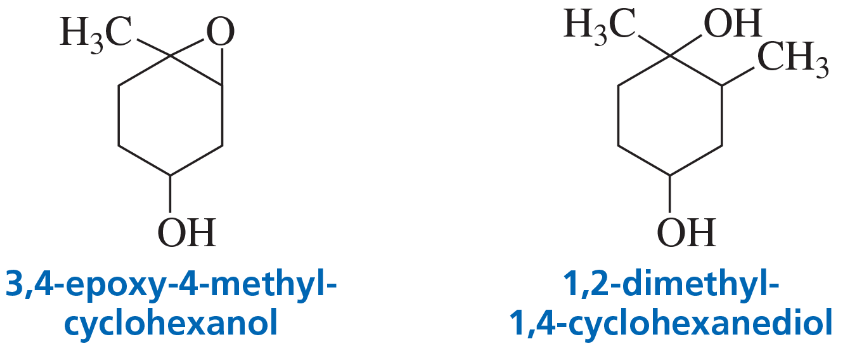
Problem 40
A student added an equivalent of 3,4-epoxy-4-methylcyclohexanol to a solution of methylmagnesium bromide in diethyl ether, and then added dilute hydrochloric acid. He expected that the product would be 1,2-dimethyl-1,4-cyclohexanediol. He did not get any of the expected product. What product did he get?
Problem 41b
Using the given starting material, any necessary inorganic reagents, and any carbon-containing compounds with no more than two carbons, indicate how each of the following compounds can be prepared:
b.
Problem 42a
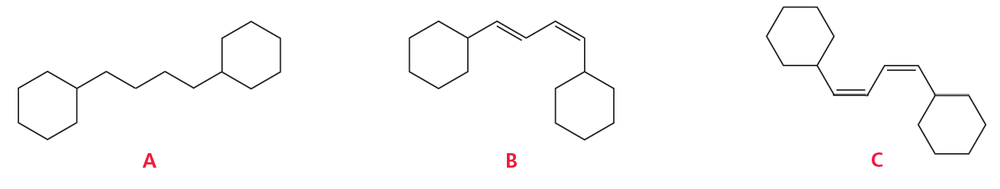
Which of the following compounds cannot be prepared by a Heck reaction?
Problem 42b
For those compounds that can be prepared by a Heck reaction, what starting materials are required?
Problem 45a
Metathesis of which of the following sets of alkenes leads to the highest yield of a single alkene?
1. 1-butene and 1-pentene
2. 2-butene and 3-hexene
3. 2-butene and 1-pentene
Problem 47
A dibromide loses only one bromine when it reacts with sodium hydroxide. The dibromide forms toluene (C6H5-CH3) when it reacts with magnesium shavings in ether followed by treatment with dilute acid. Give possible structures for the dibromide.
Problem 48a
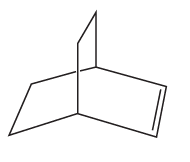
What starting material is required in order to synthesize each of the following compounds by ring-closing metathesis?
a.
Problem 49a
What product is obtained from ring-opening metathesis polymerization of each of the following compounds? (Hint: In each case, the product is an unsaturated hydrocarbon with a high molecular weight.)
a.
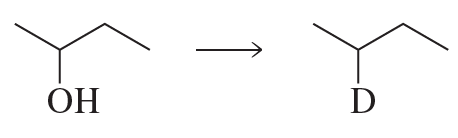
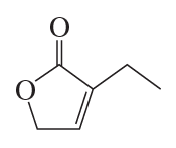
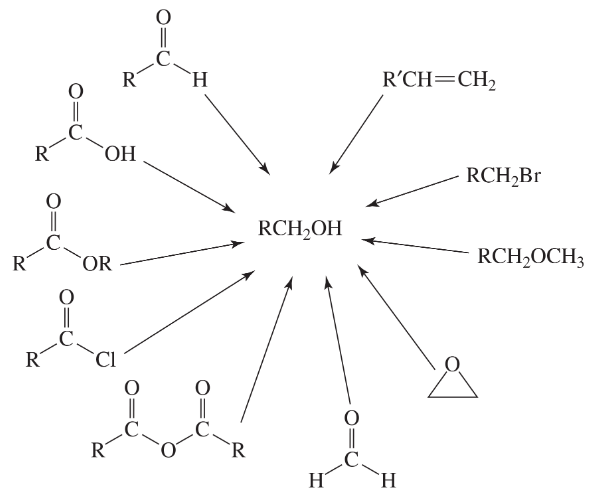
Problem 57a(6)
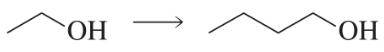
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 67d
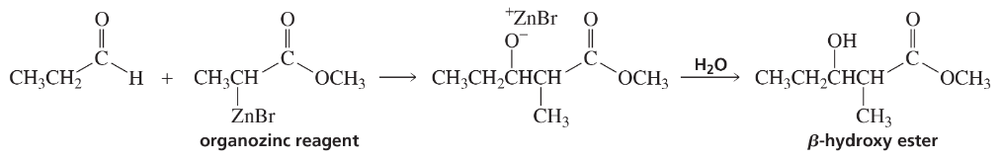
The Reformatsky reaction is an addition reaction in which an organozinc reagent is used instead of a Grignard reagent to add to the carbonyl group of an aldehyde or a ketone. Because the organozinc reagent is less reactive than a Grignard reagent, a nucleophilic addition to the ester group does not occur.
The organozinc reagent is prepared by treating an α-bromo ester with zinc.
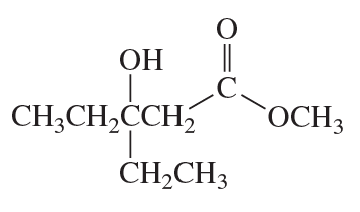
Describe how each of the following compounds can be prepared, using a Reformatsky reaction:
d.
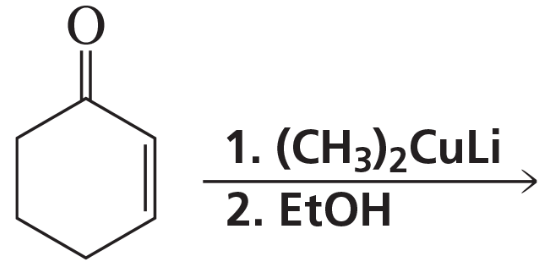
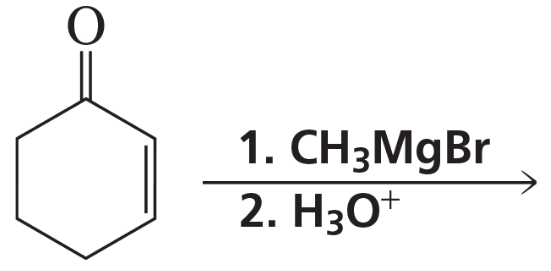
Problem 73a,b
What are the products of the following reactions? Show all stereoisomers that are formed.
a.
b.