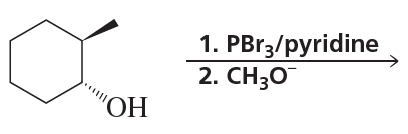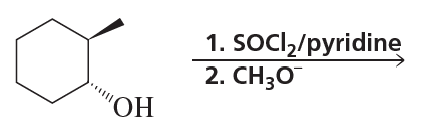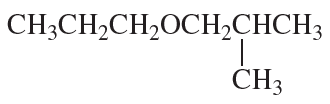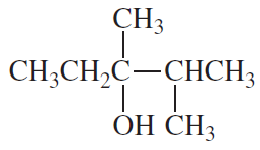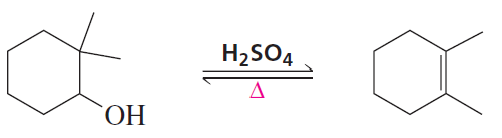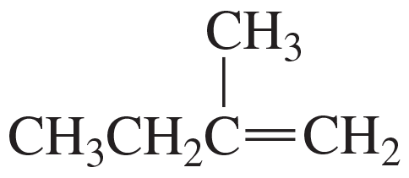Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing Compounds
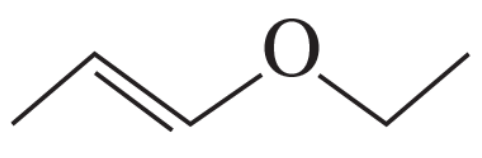
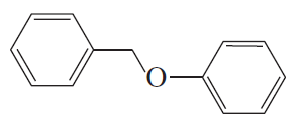
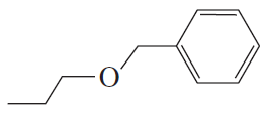
Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing Compounds- Fill in each box with the appropriate reagent: b.
Problem 10

Problem 11a
What stereoisomers do the following reactions form?
a.
Problem 11b
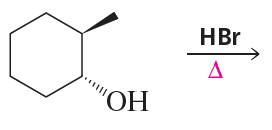
What stereoisomers do the following reactions form?
b.
Problem 11c
What stereoisomers do the following reactions form?
c.
Problem 11d
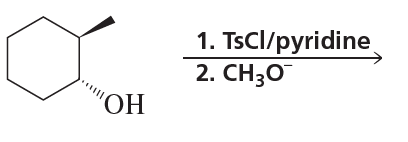
What stereoisomers do the following reactions form?
d.
Problem 12a
Show how 1-propanol can be converted into the following compounds by means of a sulfonate ester:
a. CH3CH2CH2SCH2CH3
Problem 12b
Show how 1-propanol can be converted into the following compounds by means of a sulfonate ester:
b.
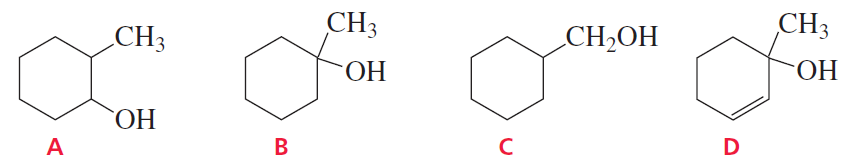
Problem 13
Which of the following alcohols dehydrates the fastest when heated with acid?
Problem 14(b)
What is the major product obtained when each of the following alcohols is heated in the presence of H2SO4?
b.
Problem 14a
What is the major product obtained when each of the following alcohols is heated in the presence of H2SO4?
a.
Problem 15a
Heating an alcohol with sulfuric acid is a good way to prepare a symmetrical ether such as diethyl ether.
a. Explain why it is not a good way to prepare an unsymmetrical ether such as ethyl propyl ether.
Problem 15b
Heating an alcohol with sulfuric acid is a good way to prepare a symmetrical ether such as diethyl ether.
b. How would you synthesize ethyl propyl ether?
Problem 16a
Propose a mechanism for each of the following reactions:
a.
Problem 18
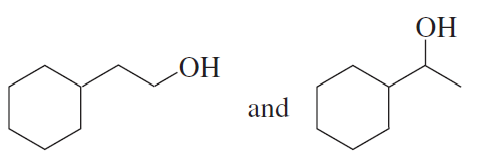
Explain why the following alcohols, when heated with acid, form the same alkene.
Problem 19a
What stereoisomers are formed in the following reactions? Which stereoisomer is the major product?
a. the acid-catalyzed dehydration of 1-pentanol to 2-pentene
Problem 19b
What stereoisomers are formed in the following reactions? Which stereoisomer is the major product?
b. the acid-catalyzed dehydration of 3,4-dimethyl-3-hexanol to 3,4-dimethyl-3-hexene
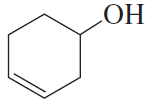
Problem 20
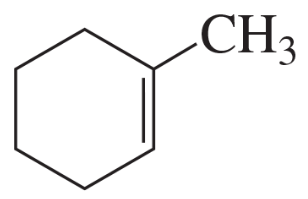
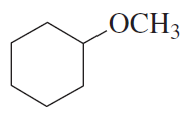
If the compound shown in the margin is heated in the presence of H2SO4,
a. what constitutional isomer would be formed in greatest yield?
b. what stereoisomer would be formed in greater yield?

Problem 21a,b
What alcohol would you treat with phosphorus oxychloride and pyridine to form each of the following alkenes?
a.
b.
Problem 22a(1,2,3)
What product is obtained from the reaction of each of the following alcohols with
a. H2CrO4?
1. 3-pentanol
2. 1-pentanol
3. 2-methyl-2-pentanol
Problem 22c(1,2,3)
What product is obtained from the reaction of each of the following alcohols with
c. the regents required for a Swern oxidation?
1. 3-pentanol
2. 1-pentanol
3. 2-methyl-2-pentanol
Problem 23
What are the major products obtained when the following ether is heated with one equivalent of HI?
Problem 24c,d
What are the major products obtained when each of the following ethers is heated with one equivalent of HI?
c.
d.
Problem 24e
What are the major products obtained when each of the following ethers is heated with one equivalent of HI?
e.
Problem 25
Explain why methyl propyl ether forms both methyl iodide and propyl iodide when it is heated with excess HI.
Problem 26
Explain why HF and HCl cannot be used to cleave ethers in an SN2 reaction.
Problem 28
Would you expect the reactivity of a five-membered ring ether such as tetrahydrofuran (Table 10.2) to be more similar to the reactivity of an epoxide or to the reactivity of a noncyclic ether? Why?
Problem 29b
How can the following compounds be prepared from 3,3-dimethyl-1-butene?
b. 3,3-dimethyl-2-butanol
Problem 30a
What products are obtained from the reaction of cyclohexene oxide with
a. methoxide ion?
Problem 30b
What products are obtained from the reaction of cyclohexene oxide with
b. methylamine?
Problem 32a,b
What stereoisomers are obtained from the reaction of each of the following alkenes with OsO4 followed by aqueous H2O2?
a. trans-2-butene
b. cis-2-butene