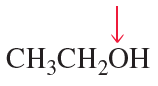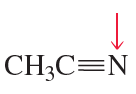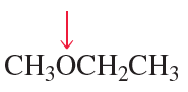Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.1 - Remembering General Chemistry:Electronic Structure and Bonding (Part 2)
Ch.1 - Remembering General Chemistry:Electronic Structure and Bonding (Part 2)Problem 47
Account for the difference in the shape and color of the potential maps for ammonia and the ammonium ion in Section 1.11.
<IMAGE>
Problem 48
If the dipole moment of CH3F is 1.847 D and the dipole moment of CD3F is 1.858 D, which is more electronegative: hydrogen or deuterium?
Problem 50a
Which of the following has a polar covalent bond?
CH3NH2 CH3CH3 CH3F CH3OH
Problem 50b
Which of the following has a bond closest to the ionic end of the bond spectrum?
CH3NH2 CH3CH3 CH3F CH3OH
Problem 51c,d
What is the hybridization of all the atoms (other than hydrogen) in each of the following?
What are the bond angles around each atom?
c. -CH3
d. ⋅CH3
Problem 51i,j
What is the hybridization of all the atoms (other than hydrogen) in each of the following?
What are the bond angles around each atom?
i. H3O+
j. H2C═O
Problem 52
Draw the condensed structure of a compound that contains only carbon and hydrogen atoms and that has
a. three sp3 hybridized carbons.
b. one sp3 hybridized carbon and two sp2 hybridized carbons.
c. two sp3 hybridized carbons and two sp hybridized carbons.
Problem 53c,d
Predict the approximate bond angles:
c. the C—N—H bond angle in (CH3)2NH
d. the C—N—C bond angle in (CH3)2NH
Problem 55b
Draw a Lewis structure for each of the following:
b. HNO2
Problem 55d
Draw a Lewis structure for each of the following:
d. NH2O−
Problem 57b
Rank the bonds from most polar to least polar.
b. C—Cl, C—I, C—Br
Problem 57c
Rank the bonds from most polar to least polar.
c. H—O, H—N, H—C
Problem 57d
Rank the bonds from most polar to least polar.
d. C—H, C—C, C—N
Problem 58b
Draw a Lewis structure for each of the following:
b. CH3OCH3
Problem 59a,b
Draw a skeletal structure for each of the compounds.
a. CH3CHO
b. CH3OCH3
Problem 59c
Draw a skeletal structure for each of the compounds.
c. CH3COOH
Problem 60a,b,c
What is the hybridization of the indicated atom in each of the following?
a.
b.
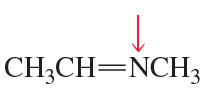
c.
Problem 60d,e,f
What is the hybridization of the indicated atom in each of the following?
d.
e.
f.
Problem 61c,d
Predict the approximate bond angles for the following:
c. the C—C—N bond angle in CH3C≡N
d. the C—C—N bond angle in CH3CH2NH2
Problem 62a,b
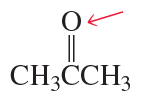
Show the direction of the dipole moment in each of the following bonds (use the electronegativities given in [Table 1.3] ):
a. H3C—Br
b. H3C—Li
Problem 65a
For each of the following molecules, indicate the hybridization of each carbon and give the approximate values of all the bond angles:
a. CH3C≡CH
Problem 65b
For each of the following molecules, indicate the hybridization of each carbon and give the approximate values of all the bond angles:
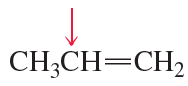
b. CH3CH═CH2
Problem 65d
For each of the following molecules, indicate the hybridization of each carbon and give the approximate values of all the bond angles:
d. CH2═CH—CH═CH2
Problem 66c
Draw a Lewis structure for each of the following:
c. (CH3)2CHCH(CH3)CH2C(CH3)3
Problem 68
Rank the following compounds from highest dipole moment to lowest dipole moment:
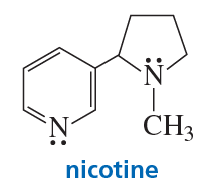
Problem 69
In which orbitals are the lone pairs in nicotine?
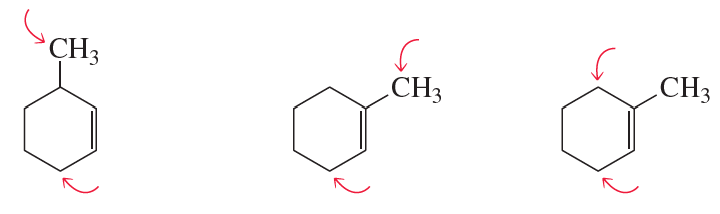
Problem 71
Do the sp2 carbons and the indicated sp3 carbons lie in the same plane?
Problem 72a
a. Which of the species have bond angles of 109.5°?
b. Which of the species have bond angles of 120°?
H2O H3O+ +CH3 BF3
Problem 72b
a. Which of the species have bond angles of 109.5°?
b. Which of the species have bond angles of 120°?
NH3 +NH4 -CH3
Problem 76
Explain why CH3Cl has a greater dipole moment than CH3F even though F is more electronegative than Cl.