 Back
BackProblem 40b
The following reaction is first order in A (red spheres) and first order in B (blue spheres): A + B → Products Rate = k[A][B]
(b) What are the relative values of the rate constant k for vessels (1)–(4)?
- Consider the first-order decomposition of A molecules (red spheres) in three vessels of equal volume. (1)-(3)
Problem 41
(c) How will the rates and half-lives be affected if the volume of each vessel is decreased by a factor of 2?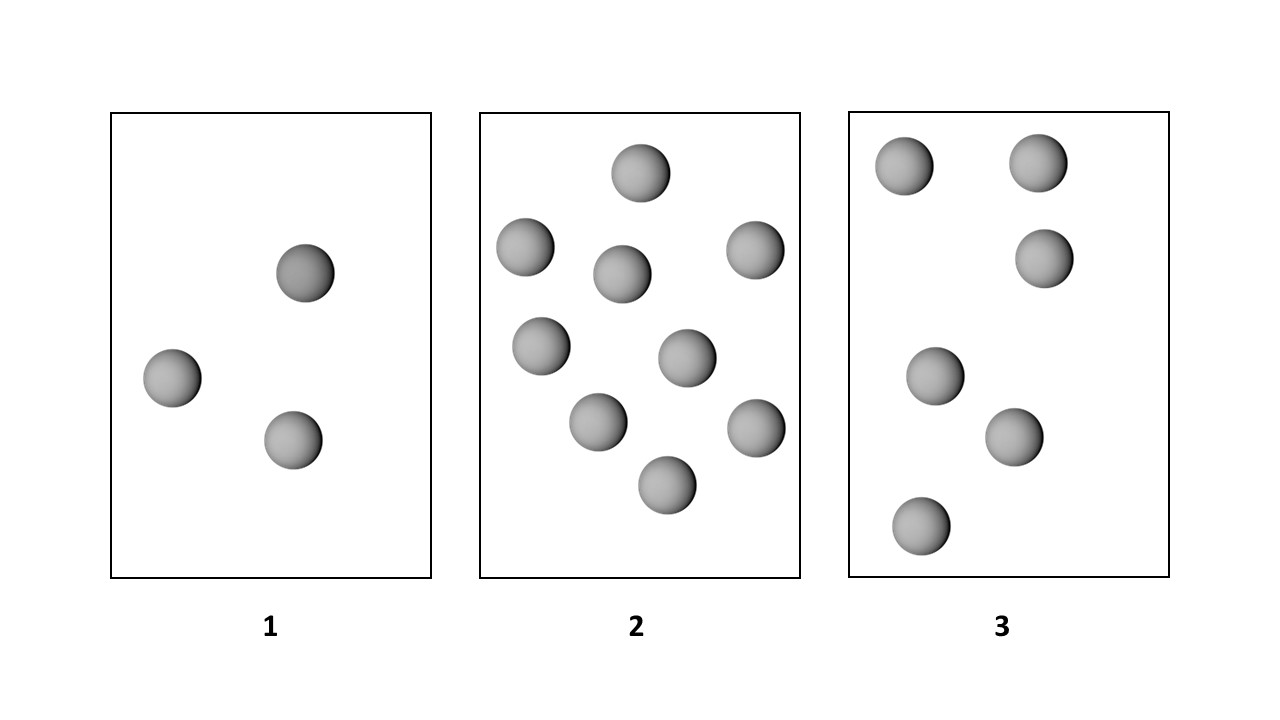
Problem 41b
Consider the first-order decomposition of A molecules (red spheres) in three vessels of equal volume. (1)-(3)
(b) What are the relative half-lives of the reactions in vessels (1)–(3)?
- Consider the first-order reaction AS B in which A molecules (red spheres) are converted to B molecules (blue spheres).
Problem 42
(a) Given the pictures at t = 0 min and t = 1 min, draw pictures that show the number of A and B molecules present at t = 2 min and t = 3 min.
Problem 43b
The following pictures represent the progress of the reaction AS B in which A molecules (red spheres) are converted to B molecules (blue spheres).
(b) Draw a picture that shows the number of A and B molecules present at t = 3 min.
Problem 43c
The following pictures represent the progress of the reaction AS B in which A molecules (red spheres) are converted to B molecules (blue spheres).
(c) Suppose that each sphere represents 6.0⨉1021 molecules and that the volume of the container is 1.0 L. What is the rate constant for the reaction in the usual units?
- The following pictures represent the progress of a reaction in which two A molecules combine to give a more complex molecule A2, 2 AS A2.
Problem 44
(b) What is the rate law?
- What is the molecularity of each of the following elementary reactions? (a)
Problem 45
(b)
(c) - The relative rates of the reaction A + B S AB in vessels (1)–(3) are 4:4:1. Red spheres represent A molecules, and blue spheres represent B molecules. (1)-(3)
Problem 46
(a) What is the order of the reaction in A and B?
Problem 48b
Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is as follows:
(b) Write structural formulas for all species present at reaction stages 1–5. Identify each species as a reactant, product, catalyst, intermediate, or transition state.
Problem 48c
Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is as follows:
(c) Which of the two steps in the mechanism is the rate-determining step? Write the rate law for the overall reaction.
Problem 48d
Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is as follows:
(d) Is the reaction endothermic or exothermic? Add labels to the diagram that show the values of the energy of reaction ΔE and the activation energy Ea for the overall reaction.
- Draw a plausible transition state for the bimolecular reaction of nitric oxide with ozone. Use dashed lines to indicate the atoms that are weakly linked together in the transition state. NO(g) + O3(g) NO2(g) + O2(g)
Problem 49

- Use the information in Table 14.1 and Figure 14.1 to estimate the instantaneous rate of appearance of NO2 at t = 350 s by calculating the average rate of appearance of NO2 over the following time intervals centered on t = 350 s. (a) 0 to 700 s (b) 100 to 600 s (c) 200 to 500 s (d) 300 to 400 s Which is the best estimate, and why?
Problem 52
Problem 54a
From the plot of concentration–time data in Figure 14.1, estimate: (a) the instantaneous rate of decomposition of N2O5 at t = 200 s.
Problem 54b
From the plot of concentration–time data in Figure 14.1, estimate: (b) the initial rate of decomposition of N2O5.
Problem 55a
From a plot of the concentration–time data in Worked Example 14.9, estimate: (a) the instantaneous rate of decomposition of NO2 at t = 100 s.
Problem 55b
From a plot of the concentration–time data in Worked Example 14.9, estimate: (b) the initial rate of decomposition of NO2.
- Ammonia is manufactured in large amounts by the reaction N21g2 + 3 H21g2S 2 NH31g2 (b) How is the rate of formation of NH3 related to the rate of consumption of N2?
Problem 56
Problem 56a
Ammonia is manufactured in large amounts by the reaction
N2(g) + 3 H2(g) → 2 NH3(g)
(a) How is the rate of consumption of H2 related to the rate of consumption of N2?
- Chlorite is reduced by bromide in acidic solution according to the following balanced equation: ClO2 -1aq2 + 4 Br-1aq2 + 4 H+1aq2S Cl-1aq2 + 2 Br21aq2 + 2 H2O1l2 (a) If Δ3Br24>Δt = 4.8 * 10-6 M>s, what is the value of Δ3ClO2 -4>Δt during the same time interval?
Problem 58
- The reaction 2NO1g2 + 2 H21g2S N21g2 + 2 H2O1g2 is first order in H2 and second order in NO. Write the rate law, and specify the units of the rate constant.
Problem 61
- Initial rate data at 25 °C are listed in the table for the reaction NH4 +1aq2 + NO2 -1aq2S N21g2 + 2 H2O1l2
Problem 66
(b) What is the value of the rate constant?

- Trimethylamine and chlorine dioxide react in water in an electron transfer reaction to form the trimethylamine cation and chlorite ion: 1CH323 N1aq2 + ClO21aq2 + H2O1l2S 1CH323 NH+1aq2 + ClO2 -1aq2 + OH-1aq2 Initial rate data obtained at 23 °C are listed in the following table.
Problem 69

