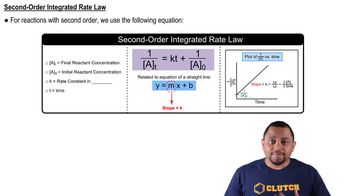
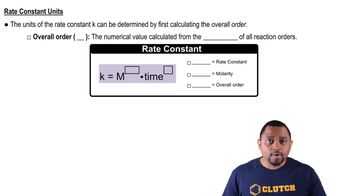
Integrated Rate Law for Second-Order Reactions
The integrated rate law for a second-order reaction can be expressed as 1/[A] = 1/[A]₀ + kt, where [A] is the concentration at time t, [A]₀ is the initial concentration, k is the rate constant, and t is the time elapsed. This equation allows us to determine the concentration of the reactant at any given time, making it essential for solving problems related to concentration changes in second-order kinetics.

 Verified step by step guidance
Verified step by step guidance