Textbook Question
Ammonia is manufactured in large amounts by the reaction
N2(g) + 3 H2(g) → 2 NH3(g)
(a) How is the rate of consumption of H2 related to the rate of consumption of N2?

 Verified step by step guidance
Verified step by step guidance
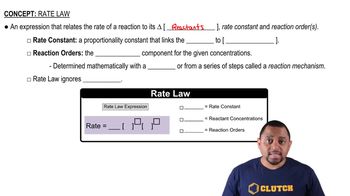


Ammonia is manufactured in large amounts by the reaction
N2(g) + 3 H2(g) → 2 NH3(g)
(a) How is the rate of consumption of H2 related to the rate of consumption of N2?