From the plot of concentration–time data in Figure 14.1, estimate: (b) the initial rate of decomposition of N2O5.

Use the information in Table 14.1 and Figure 14.1 to estimate the instantaneous rate of appearance of NO2 at t = 350 s by calculating the average rate of appearance of NO2 over the following time intervals centered on t = 350 s. (a) 0 to 700 s (b) 100 to 600 s (c) 200 to 500 s (d) 300 to 400 s Which is the best estimate, and why?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Instantaneous Rate vs. Average Rate
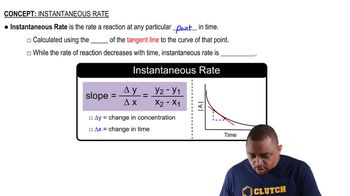
Rate of Reaction

Data Interpretation from Graphs and Tables
Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is as follows:
(c) Which of the two steps in the mechanism is the rate-determining step? Write the rate law for the overall reaction.
Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is as follows:
(d) Is the reaction endothermic or exothermic? Add labels to the diagram that show the values of the energy of reaction ΔE and the activation energy Ea for the overall reaction.
From the plot of concentration–time data in Figure 14.1, estimate: (a) the instantaneous rate of decomposition of N2O5 at t = 200 s.
From a plot of the concentration–time data in Worked Example 14.9, estimate: (a) the instantaneous rate of decomposition of NO2 at t = 100 s.
