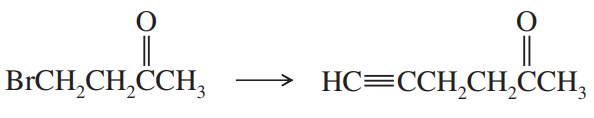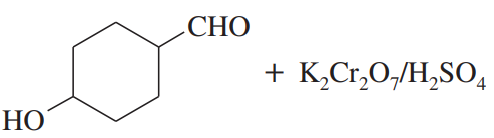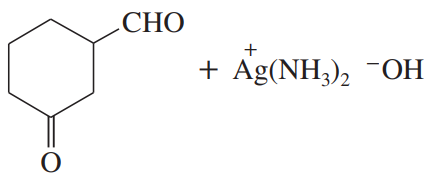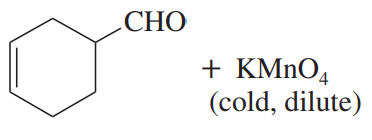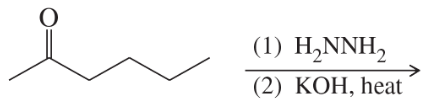Back
BackProblem 23f
Show what amines and carbonyl compounds combine to give the following derivatives.
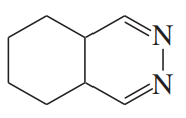
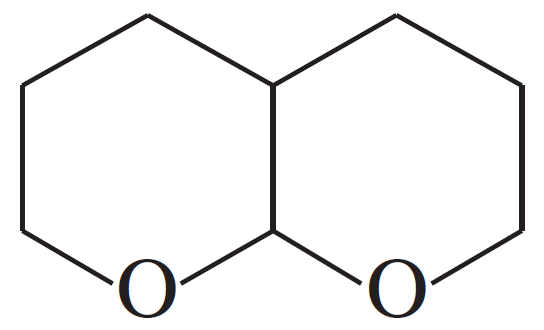
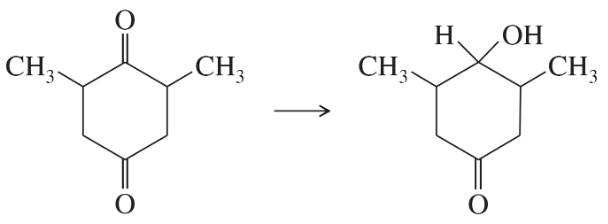
(f)
Problem 24
Propose a mechanism for the acid-catalyzed reaction of benzaldehyde with methanol to give benzaldehyde dimethyl acetal.
Problem 25
Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone dimethyl acetal.
Problem 26a,b,c
Show what alcohols and carbonyl compounds give the following derivatives.
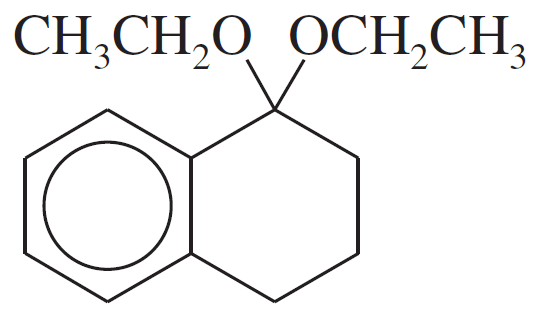
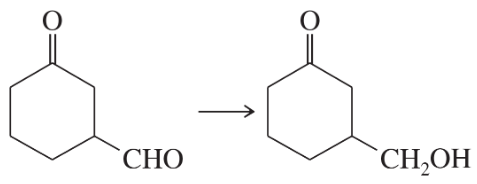
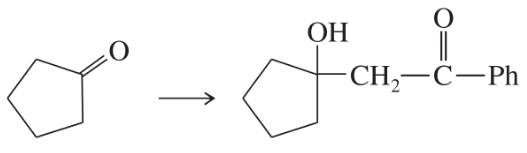
(a)
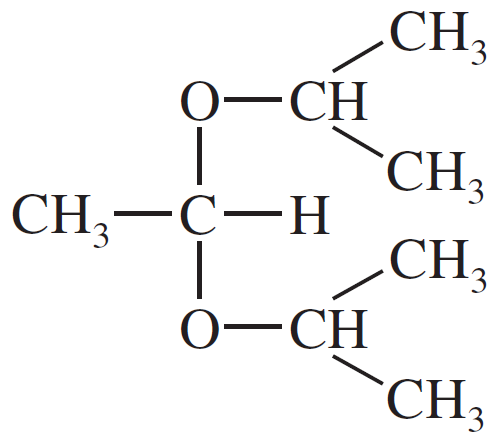
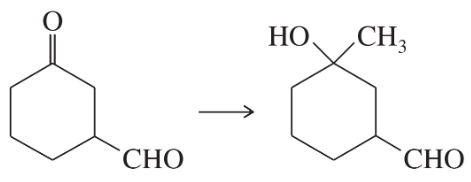
(b)
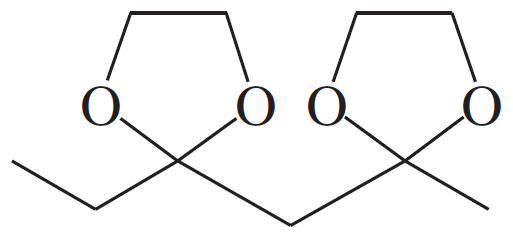
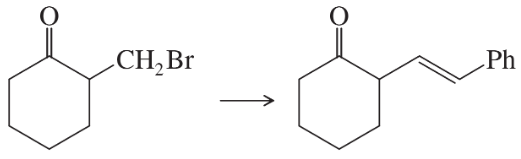
(c)
Problem 27
In the mechanism for acetal hydrolysis shown, the ring oxygen atom was protonated first, the ring was cleaved, and then the methoxy group was lost. The mechanism could also be written to show the methoxy oxygen protonating and cleaving first, followed by ring cleavage. Draw this alternative mechanism.
Problem 28a
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with ethylene glycol to give cyclohexanone ethylene acetal.
Problem 28b
Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone ethylene acetal.
Problem 28d
Propose a mechanism for the acid-catalyzed hydrolysis of the acetal given in Problem 18-26(f).
Problem 29a
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(a)
Problem 29b
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(b)
Problem 29c
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(c)
Problem 29d
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(d)
Problem 29e
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(e)
Problem 29f
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need.
(f)
Problem 30
Trimethylphosphine is a stronger nucleophile than triphenylphosphine, but it is rarely used to make ylides. Why is trimethylphosphine unsuitable for making most phosphorus ylides?
Problem 30a
Write the sequence of steps required for the conversion of benzene into benzenediazonium chloride.
Problem 31a
Like other strong nucleophiles, triphenylphosphine attacks and opens epoxides. The initial product (a betaine) quickly cyclizes to an oxaphosphetane that collapses to an alkene and triphenylphosphine oxide.
(a) Show each step in the reaction of trans-2,3-epoxybutane with triphenylphosphine to give but-2-ene. What is the stereochemistry of the double bond in the product?
Problem 31b
Like other strong nucleophiles, triphenylphosphine attacks and opens epoxides. The initial product (a betaine) quickly cyclizes to an oxaphosphetane that collapses to an alkene and triphenylphosphine oxide.
(b) Show how this sequence might be used to convert cis-cyclooctene to trans-cyclooctene.
Problem 32
(a) Outline the syntheses indicated in Solved Problem 18-2, beginning with aldehydes and alkyl halides.
(b) Both of these syntheses of 1-phenylbuta-1,3-diene form the central double bond. Show how you would synthesize this target molecule by forming the terminal double bond.
Problem 33a,b
Show how Wittig reactions might be used to synthesize the following compounds. In each case, start with an alkyl halide and a ketone or an aldehyde.
(a) Ph–CH=C(CH3)2
(b) Ph–C(CH3)=CH2
Problem 33c,d
Show how Wittig reactions might be used to synthesize the following compounds. In each case, start with an alkyl halide and a ketone or an aldehyde.
(c) Ph–CH=CH–CH=CH–Ph
(d)
Problem 34a
Predict the major products of the following reactions.
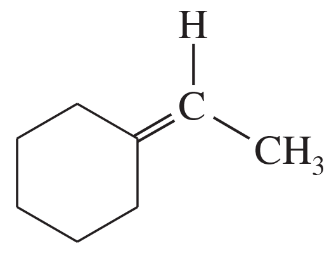
(a)
Problem 34b
Predict the major products of the following reactions.
(b)
Problem 34c
Predict the major products of the following reactions.
(c)
Problem 34d
Predict the major products of the following reactions.
(d)
Problem 35a
Propose a mechanism for both parts of the Wolff–Kishner reduction of cyclohexanone: the formation of the hydrazone, and then the base-catalyzed reduction with evolution of nitrogen gas.
Problem 35b
Propose a mechanism for both parts of the Wolff–Kishner reduction of cyclohexanone: the formation of the hydrazone, and then the base-catalyzed reduction with evolution of nitrogen gas.
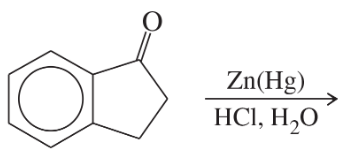
Problem 36a
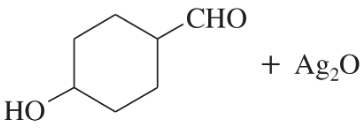
Predict the major products of the following reactions:
(a)
Problem 36b
Predict the major products of the following reactions:
(b)
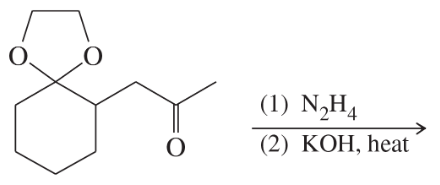
Problem 36c
Predict the major products of the following reactions:
(c)