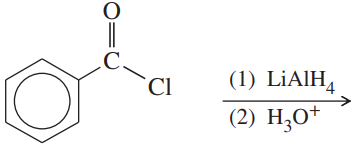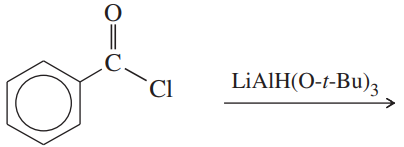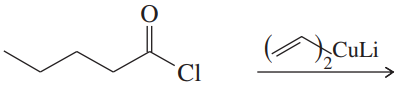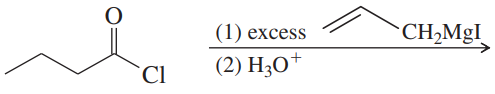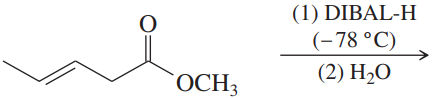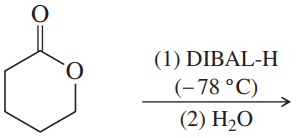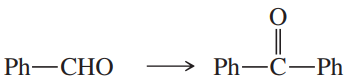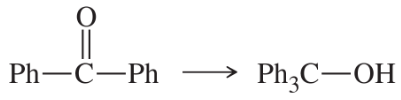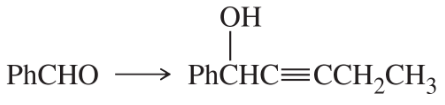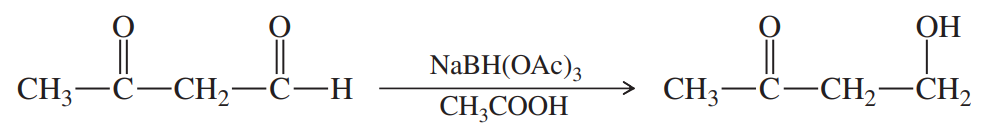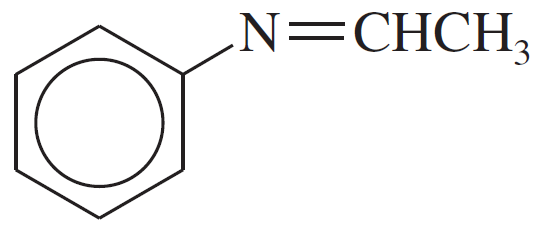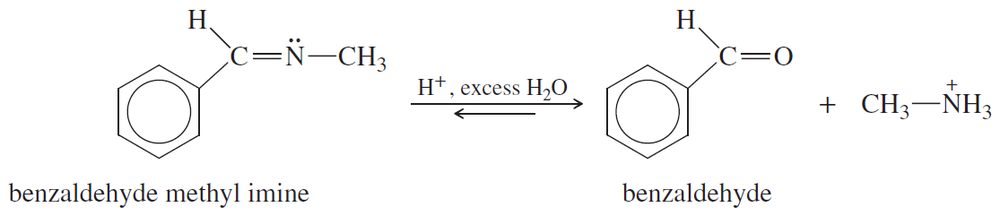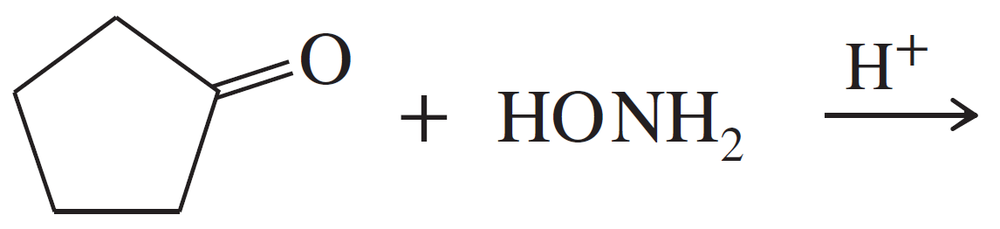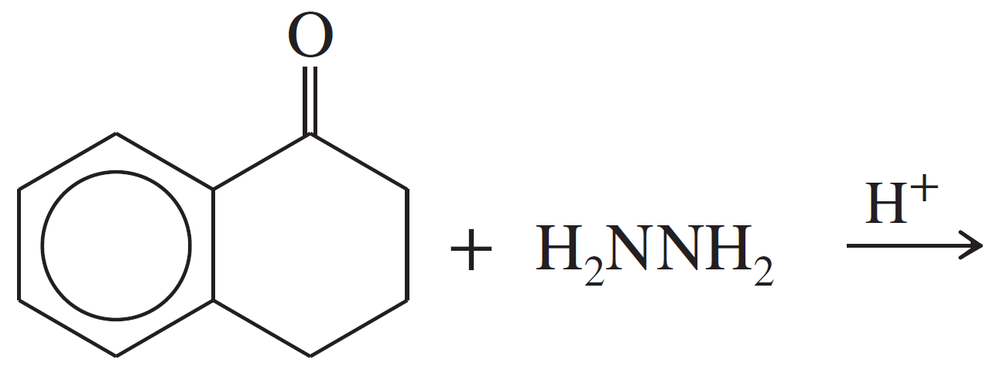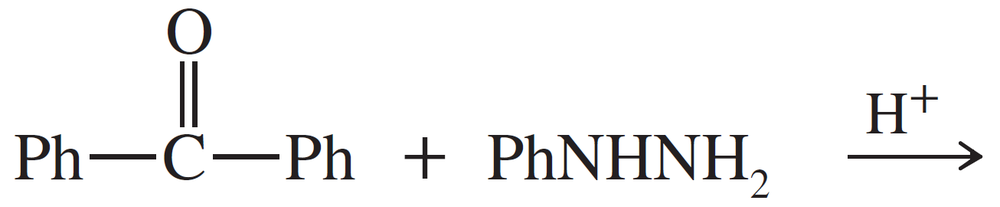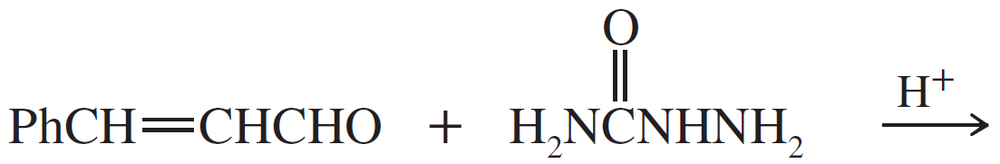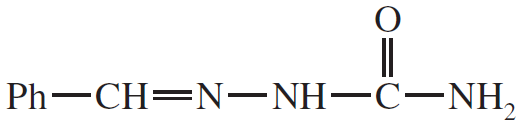Back
BackProblem 8d
Predict the products of the following reactions.
(d) product of (c) + cyclopentylmagnesium bromide, then acidic hydrolysis
Problem 8e
Predict the products of the following reactions.
(e) product of (c) + DIBAL-H, then hydrolysis
Problem 9a,b
Show how the following transformations may be accomplished in good yield. You may use any additional reagents that are needed.
(a) bromobenzene → propiophenone
(b) CH3CH2CN → heptan-3-one
Problem 9c,d
Show how the following transformations may be accomplished in good yield. You may use any additional reagents that are needed.
(c) benzoic acid → phenyl cyclopentyl ketone
(d) 1-bromohept-2-ene → oct-3-enal
Problem 10a,b
Predict the products of the following reactions:
(a)
(b)
Problem 10c,d
Predict the products of the following reactions:
(c)
(d)
Problem 10e,f
Predict the products of the following reactions:
(e)
(f)
Problem 11a
Show how you would accomplish the following synthetic conversions. You may use any additional reagents and solvents you need.
(a)
Problem 11b
Show how you would accomplish the following synthetic conversions. You may use any additional reagents and solvents you need.
(b)
Problem 11d
Show how you would accomplish the following synthetic conversions. You may use any additional reagents and solvents you need.
(d)
Problem 12a
Sodium triacetoxyborohydride, NaBH(OAc)3, is a mild reducing agent that reduces aldehydes much more quickly than ketones. It can be used to reduce aldehydes in the presence of ketones, such as in the following reaction:
(a) Draw a complete Lewis structure for sodium triacetoxyborohydride.
Problem 12b
Sodium triacetoxyborohydride, NaBH(OAc)3, is a mild reducing agent that reduces aldehydes much more quickly than ketones. It can be used to reduce aldehydes in the presence of ketones, such as in the following reaction:

(b) Propose a mechanism for the reduction of an aldehyde by sodium triacetoxyborohydride.
Problem 13a
Propose mechanisms for
(a) the acid-catalyzed hydration of chloral to form chloral hydrate.
Problem 13b
Propose mechanisms for
(b) the base-catalyzed hydration of acetone to form acetone hydrate.
Problem 15
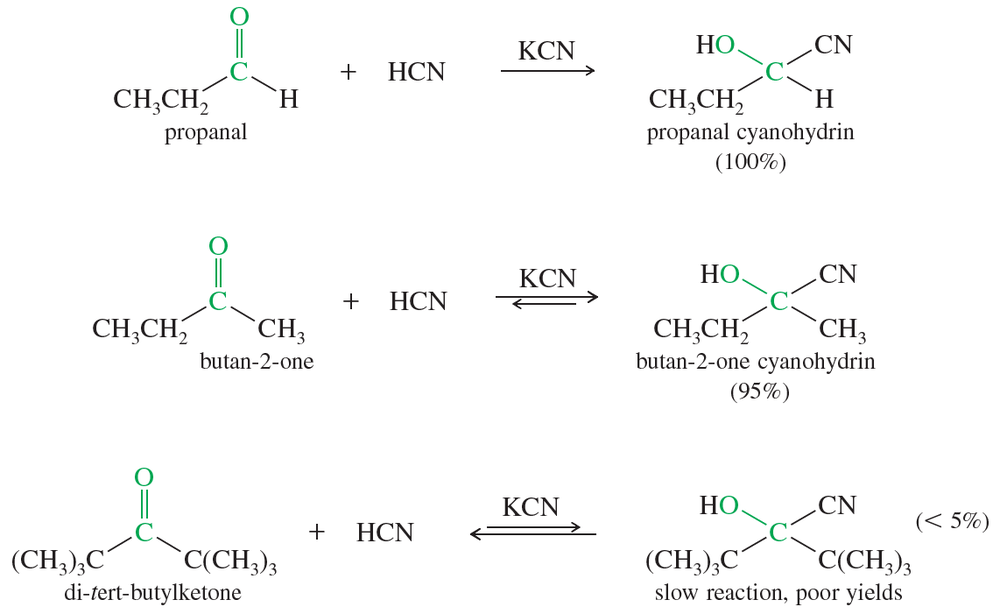
Propose a mechanism for each cyanohydrin synthesis just shown.
Problem 16a
Show how you would accomplish the following syntheses.
(a) acetophenone → acetophenone cyanohydrin
Problem 16b
Show how you would accomplish the following syntheses.
b. cyclopentanecarbaldehyde → 2-cyclopentyl-2-hydroxyacetic acid
Problem 16c
Show how you would accomplish the following syntheses.
c. hexan-1-ol → 2-hydroxyheptanoic acid
Problem 17
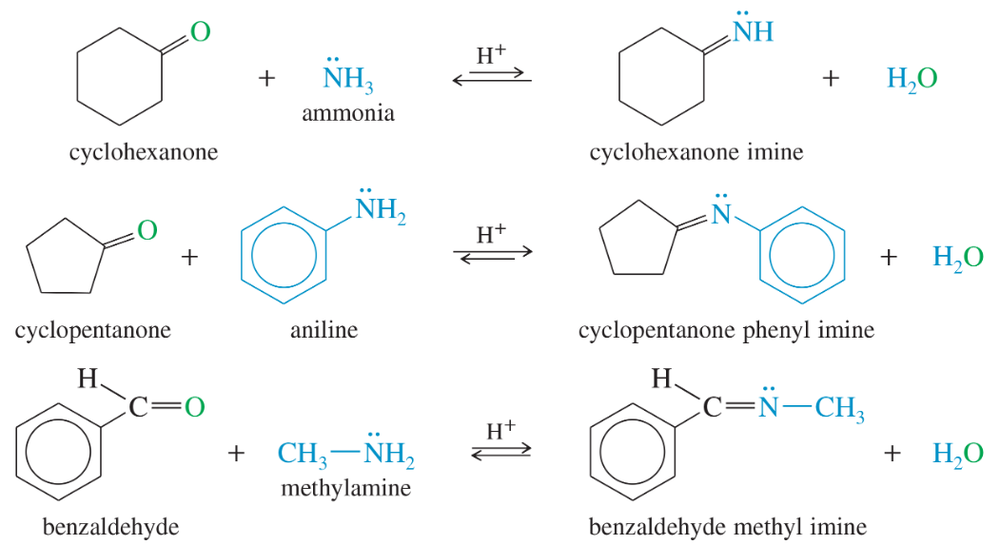
Propose mechanisms for the three imine-forming reactions just shown.
Problem 18a
Depending on the reaction conditions, two different imines of formula C8H9N might be formed by the reaction of benzaldehyde with methylamine. Explain, and give the structures of the two imines.
Problem 18d
Show how hex-1-yne might be converted to
d. 1,1,2,2-tetrabromohexane.
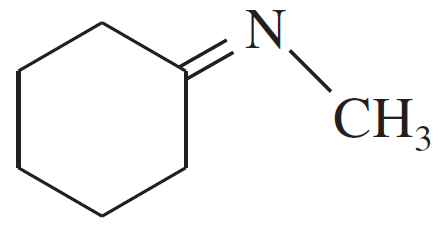
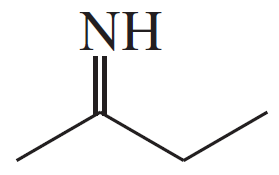
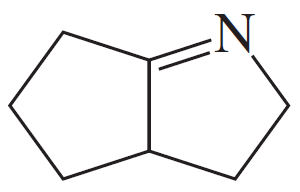
Problem 19a,b,c
Give the structures of the carbonyl compound and the amine used to form the following imines.
(a)
(b)
(c)
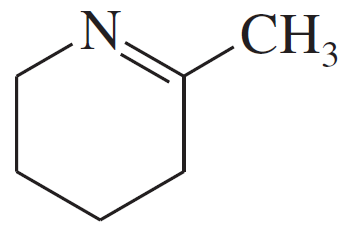
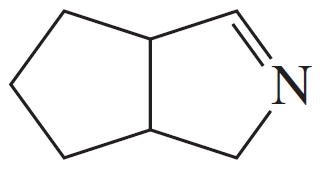
Problem 19d,e,f
Give the structures of the carbonyl compound and the amine used to form the following imines.
(d)
(e)
(f)
Problem 20a
Propose a mechanism for the hydrolysis of benzaldehyde methyl imine just shown.
Problem 21a
2,4-Dinitrophenylhydrazine is frequently used for making derivatives of ketones and aldehydes because the products (2,4-dinitrophenylhydrazones, called 2,4-DNP derivatives) are even more likely than the phenylhydrazones to be solids with sharp melting points. Propose a mechanism for the reaction of acetone with 2,4-dinitrophenylhydrazine in a mildly acidic solution.
Problem 22a,b
Predict the products of the following reactions.
(a)
(b)
Problem 22c,d
Predict the products of the following reactions.
(c)
(d)
Problem 23a,b,c
Show what amines and carbonyl compounds combine to give the following derivatives.
(a)
(b)
(c)
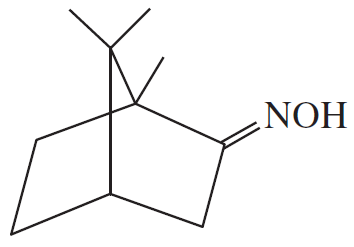
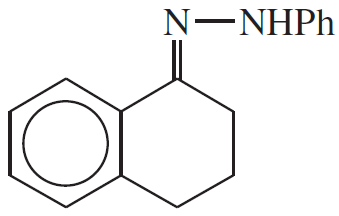
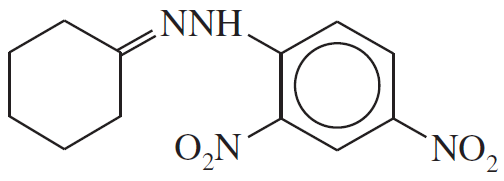
Problem 23d
Show what amines and carbonyl compounds combine to give the following derivatives.
(d)
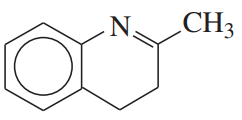
Problem 23e
Show what amines and carbonyl compounds combine to give the following derivatives.
(e)